Cytotoxic chemotherapy, or systemic anti-cancer therapy (SACT), is considered hazardous to healthcare workers. Due to significant toxicities associated with exposure, they are classified as hazardous drugs (HDs) (Connor et al, 2016; O'Connor et al, 2022). Acute symptoms such as nausea, eye irritation, headaches and rashes, and serious chronic reproductive problems including miscarriages and infertility have been documented for more than four decades (Hemminki et al, 1985; Valanis et al, 1993; Valanis et al, 1999; Ratner et al, 2010; Lawson et al, 2012; Couch et al, 2013; Momeni et al, 2013; Connor et al, 2014; Elshaer, 2017; Alehashem and Baniasadi, 2018; Baniasadi et al, 2018; Nassan et al, 2021). Following a landmark 1979 paper that demonstrated mutagenic substances in the urine of nurses involved with the handling of chemotherapy (Falck et al, 1979), safe handling guidelines were published by the then American Society of Hospital Pharmacists (now known as the American Society of Health-System Pharmacists) (Stolar and Power, 1983). Subsequent safety guidelines emerged from other organizations including the Oncology Nursing Society (ONS), the European Oncology Nursing Society (EONS) and the Infusion Nursing Society (INS) (Polovich and Olsen, 2018; EONS, 2019; Gorski et al, 2021). Nursing-specific components of these guidelines include required education for all staff who may come in contact with hazardous drugs, the use of personal protective equipment (PPE) for preparation, administration and disposal, and handling of excreta, and most recently, the use of closed system drug-transfer devices (CSTDs).
The ability to damage chromosomes is a major area of concern with hazardous drug exposure. In a comprehensive meta-analysis of 17 studies comparing nurses and pharmacists working with cytotoxic chemotherapy with those who did not, a statistically significant correlation between drug exposure and DNA damage was shown (P<0.001) (Roussel et al, 2019). In addition, the International Agency on Research on Cancer (2022) has identified several commonly used drugs as being carcinogenic, and indeed, secondary malignancies have been an ongoing concern for patients receiving cancer treatment (Olsen et al, 2019).
Inadvertent environmental contamination from leakage and drips during administration can spread throughout the healthcare environment. This contamination can be transferred to areas such as counter tops, computer keyboards, and computer mice (Ndaw et al, 2018; Chauchat et al, 2019; Salch et al, 2019; Friese et al, 2020). This is a significant cause for concern as dermal contact can lead to absorption and subsequent urinary excretion that can affect not only nurses but all personnel working in the facility (Hon et al, 2014; Friese et al, 2015; Hon et al, 2015; Ndaw et al, 2018).
One of the largest wipe test studies was performed in 51 Canadian hospitals and demonstrated that half of the 584 samples were positive for cyclophosphamide, including 91% of the infusion chair armrests (Janes et al, 2015). Similarly, French researchers tested for the presence of several chemotherapy agents in 11 hospitals and found widespread contamination in work areas that included computer keyboards and telephones. Even more concerning, 63% of the urine samples collected from the healthcare workers were positive for at least one drug (Ndaw et al, 2018). The percentage of nurses who tested positive (55%) was greater than the percentage of pharmacists (45%), underscoring the unique vulnerabilities faced by nurses during drug administration and disposal. Exposure problems have been reported in other European countries. Results of an inspection in Poland in 2010 revealed that 3220 healthcare workers were exposed to cytotoxic chemotherapy, 92% of which were female (Palaszewska-Tkacz et al, 2019). Because the chronic long-term side effects of exposure require long-term longitudinal studies, there are limited data showing direct cause and effect. However, chromosomal damage has been shown to occur with hazardous drug exposure (Roussel et al, 2019), and is one of the hallmarks of carcinogenesis.
Nursing vulnerability
Safe compounding of chemotherapy has historically received more attention than drug administration (Santillo et al, 2018), and guidelines for nursing safety vary considerably across Europe. Depending on the country, compliance is voluntary (Mathias et al, 2019). The process of administering cytotoxic drugs is inherently prone to result in environmental contamination and exposure (Eisenberg, 2016; Marler-Hausen et al, 2020). Unlike the sequential nature of compounding—ideally performed in a sequestered pharmacy environment using a specialized biologic safety cabinet or isolator—drug administration occurs in unprotected common areas with infusion chairs or hospital beds connected by hallways. Nurses are subject to multitasking and frequent interruptions, not only by the patient and family members, but also by staff and/or other assigned patients. Both the physical layout and frequent interruptions can lead to spills or drips that are not easily contained (Eisenberg, 2016; DeJoy et al, 2017). One wipe test study found that 78% of the nursing areas were positive compared with 55% in the pharmacy (Bartel et al, 2018).
The use of properly designed PPE is important in preventing dermal absorption of chemotherapy (Polovich and Olsen, 2018; Santillo et al, 2018). However, surveys indicate that compliance with PPE during administration and disconnection is inconsistent and less than that found during compounding activities, irrespective of country (Polovich and Martin, 2011; Momeni et al, 2013; Boiano et al, 2014; 2015; Graeve et al, 2017; He et al, 2017). This is partially due to educational deficiencies, a perceived decreased risk during administration and disconnection of intravenous (IV) tubing, and organisational safety culture and staffing concerns (Polovich and Clark, 2012; Simons and Toland, 2017).
A recent survey by EONS showed that 38% of the surveyed nurses adhered to safety guidelines, and only 16% used the proper chemotherapy gown (Ullgren et al, 2021). Respondents indicated a general lack of knowledge and training as well as peer pressure. The results of another survey of French oncology nurses in 2020 were presented during the 2021 virtual EONS conference (Dielenseger, 2021). Of the 129 respondents, 87% agreed there were risks involved with handling chemotherapy but 79% stated their level of knowledge about contamination studies was weak or poor; 31% stated their knowledge of safe handling practices was poor, and 38% had not received specific training. Less than 20% wore a gown for protection. Perhaps most concerning, almost 70% of the nurses were between 21 and 45 years of age, highlighting the potential impact for reproductive concerns.
Although an essential component of safety, PPE does not stop chemotherapy from escaping into the environment during priming, connecting and disconnecting the IV administration set (Eisenberg, 2016; 2017; Polovich and Olsen, 2018). Therefore, the first priority in hazardous drug safety is preventing drugs from getting into the environment as studies have shown that contamination also affects other hospital staff who are not required to wear PPE for their duties such as hospital assistants or ward secretaries (Hon et al, 2014; Ndaw et al, 2018). Furthermore, it has been shown that hazardous drug contamination tends to remain on surfaces for prolonged periods and is difficult to remove (Anderson et al, 2001; Roberts et al, 2006; Anastasi et al, 2015; Böhlandt et al, 2015).
Due to hydrostatic pressure, IV tubing is subject to dripping after being disconnected from the patient (Eisenberg, 2018). Connor et al (2010) studied contamination in 3 major cancer centres in the USA and found 43% of the nursing areas were positive for at least one hazardous drug. The highest drug concentration was discovered on the lid of a disposal container, most likely from the dripping tubing. A large study of 83 Canadian cancer centres showed that a staggering 81% of the armrests were contaminated (Chauchat et al, 2019). Another study also demonstrated a high concentration of hazardous drug on a waste bin cover, and noted that 39% of nursing hand wipes were positive for hazardous drug residue (Labrèche et al, 2021). Even after being flushed with a neutral solution, drug residue is still present in the IV administration set and can result in both contamination and exposure (Claraz et al, 2020).
Bedside spills are another source of contamination and is likely under-reported. One large survey from the USA revealed that 12% of the nurses admitted to having had a spill within a week of completing the survey (Boiano et al, 2014). Exposure occurs when IV bags are spiked and unspiked, particularly if the IV bag is accidentally punctured at the bedside (Vandenbroucke and Robays, 2001). This practice continues to be commonplace in parts of Europe (Simons and Toland, 2015; Field et al, 2017) and was singled out as a specific concern in the Guidance on Handling of Injectable Cytotoxic Drugs in Clinical Areas in NHS Hospitals in the UK (Santillo et al, 2018).
Closed system drug-transfer devices (CSTDs)
Since a number of situations can result in nursing exposure during administration of antineoplastic chemotherapy, focus should be placed on eliminating or minimising the likelihood of drug escaping from the IV infusion bag, syringe or administration set. In 1999, Sessink and colleagues described a successful compounding test using the first closed system drug-transfer device (CSTD) (referred to in the article as a ‘hazardous drug containment system’) (Sessink et al, 1999). Acknowledging that vertical flow biologic safety cabinets alone were not sufficient in preventing contamination during compounding, the device had been developed to protect the pharmacist during compounding of chemotherapy and other hazardous drugs but could also be adapted for administration (Sessink and Bos, 1999; Connor et al, 2002).
The fundamental parts of all CSTDs include a vial adaptor that prevents aerosols and vapours from escaping, and a connecting component attached to a syringe for transferring the drug into the IV bag. This syringe component can also be used for administration, although devices with a membrane-to-membrane design require the addition of a Luer adaptor. In 2004, the National Institute for Occupational Safety & Health (NIOSH) published its formal CSTD definition based on the only product available at that time (NIOSH, 2004). Figure 1 represents a typical membrane-to-membrane CSTD design. Other CSTDs were subsequently developed, with some using a Luer design (Massoomi, 2015). This placed a higher emphasis on drug administration since Luer adaptors were not required for the administration of hazardous drugs, helping to minimise the impact to nursing workflow and reducing the number of individual components.
A plethora of studies have demonstrated the benefit of various CSTDs. Although many have focused on compounding, a 2021 French study specifically examined potential nursing contamination by comparing 5-fluorouracil (5-FU) administered by one of three methods: Luer connection, needle-free Luer device, and a CSTD. A statistically significant difference (P=0.010) in contamination reduction was seen using the CSTD compared with either of the other administration methods (Pesqué et al, 2021).
In the USA, most hospital decisions regarding CSTDs are made by the pharmacy department, although a more collaborative effort between nursing and pharmacy has been advocated (Eisenberg, 2012). Drug administration is very different from compounding, which can impact how devices are designed and used. The general exclusion of nursing staff from product selection occurs because, historically, CSTDs were pharmacy-centric devices and therefore marketed to pharmacists with less focus on the specific needs of nurses at the bedside. Only recently have oncology nurses in the USA become more familiar with the abbreviation, in part due to a relatively new pharmacy standard that has had significant implications for nurses. USP General Chapter 800 (USP 800) was published by the US Pharmacopeia in 2016 and provides standards for both compounding and administration of HDs (US Pharmacopeia, 2016). It marked the first time that a pharmacy-based organisation dictated practice for nurses (Eisenberg, 2017). Although the chapter recommends CSTDs for compounding, it requires them for administration in the USA—again acknowledging the unique risks faced by nurses. However, despite the requirement, implementation at the bedside has posed challenges due to the unique nature of drug administration; what may work well in the pharmacy may not work as well at the bedside (Eisenberg, 2018).
The use of CSTDs during compounding varies widely throughout Europe. In the UK, for example, it is common for cytotoxic chemotherapy to be compounded without them—particularly for drugs prepared in syringes. This places responsibility of using a CSTD (if available) on the bedside nurse. In the previously described survey of 129 French oncology nurses, only 12% reported using a CSTD for administration (Dielenseger, 2021).
Terminology confusion
Although the acronym CSTD has found its way into the lexicon of some nurses in the USA, in Europe there is confusion between the terms ‘closed systems’ and ‘closed system transfer devices’, with the former referring to needleless Luer connectors (Field et al, 2017). Needleless connectors (eg, Bionector, Q-Syte, Clave) began emerging in the 1990s as a method of preventing needle-stick injuries and subsequent HIV transmission (Hadaway, 2012). However, these connectors alone were not designed to prevent leakage of chemotherapy (Marler-Hausen et al, 2020) and are only attached to one side of a Luer connection—leaving the IV administration set or syringe vulnerable to leakage.
To highlight this endemic misunderstanding, 44% of the nurses in a recent UK survey (n=55) responded that a closed system was used in administration. The authors postulated that the term may have different meanings for different nurses (Simons and Toland, 2017) as bedside use of CSTDs is not a common practice in the UK (Marler-Hausen et al, 2020). This same survey also showed that 46% of the respondents experienced adverse side effects, which included headache, dizziness, nausea, alopecia, and reproductive issues—the same symptoms described in historic and current studies where CSTDs and proper PPE are not in use (Lawson et al, 2012; Elshaer, 2017; Nassan et al, 2021). A recent multi-country EONS survey (n=616) reported that 67% of the nurses always used a CSTD for administration although again there is doubt over whether the term was fully understood (Ullgren et al, 2021). The term ‘closed’ can mean different things depending on the context and the training of the nurse, and is used in many aspects of IV therapy, phlebotomy, and general medical surgical nursing practice.
To further illustrate the confusion, two nursing articles describing safer methods of administering chemotherapy have been published. One uses the term ‘cytotoxic safe infusion systems’ (CSIS) whereas the other discusses ‘safe infusion devices’ (SIDs) (Lalande et al, 2015; Forges et al, 2021). However, neither of these systems provide the safety of a CSTD, nor were ‘closed system transfer devices’ even mentioned in the articles.
Closed safety system for administration (CSSA) proposal
In an effort to decrease misunderstanding and emphasise devices specifically engineered to protect the nurse, the term ‘closed safety system for administration’ (CSSA) has been proposed by CytoPrevent. The CytoPrevent board consists of experts from several countries: scientists, pharmacists, doctors and nurses who are dedicated to increasing protection for healthcare workers during handling, preparing, or administering cytotoxic drugs (Box 1). This multidisciplinary initiative was launched in April 2020 to raise awareness among European policymakers and recommend new measures to prevent contamination risks or to decrease them to ‘as low as reasonably achievable’, a principle used in radiation safety. Initially chaired by Dr Paul Sessink, whose expertise in hazardous drug environmental wipe sampling and safety dates back more than two decades, the group's mission is to increase awareness of hazardous drugs and to promote safety for healthcare workers throughout Europe. CytoPrevent (https://cytoprevent.eu) is also working to strengthen the Carcinogen and Mutagen Directive (CMD) 2004/37/EC of the European Parliament and of the Council of 29 April 2004 on the protection of workers from the risks related to exposure to carcinogens or mutagens at work. Directive (EU) 2022/431, issued in March 2022, amended 2004/37/EC and is closely tied to Europe's occupational safety and health (OSH) guidelines.
Box 1.Cytoprevent board membersJohan Vandenbroucke (Chairman): Pharmacist, Belgium, past president of the International Society of Oncology Pharmacy PractitionersMatthew Fowler: Advanced Clinical Practitioner in Oncology and Haematology, University Hospitals Birmingham, UK and European Oncology Nursing Society MemberHélène Levert: Hospital Pharmacist at University Hospital Saint-Louis in Paris (AP-HP), FranceAlison Simons: Oncology Nurse and Senior Lecturer at Birmingham City University, UKSamantha Tolan: Cancer Haemato-oncology Nurse and Senior Lecturer, School of Nursing and Midwifery, Birmingham, UKLuis Mazon: Risk Prevention Department, Fuenlabrada University Hospital, Madrid, SpainFred Massoomi: Compounding Compliance Director, Option Care Health, USASeth Eisenberg: Oncology Nurse, International Lecturer, Professional Practice Coordinator, Infusion Service, Seattle Cancer Care Alliance Ambulatory Clinic, USA
It is important to note that none of the board members are employed by device manufacturers. However, it is believed that separating CSSAs from CSTDs will help protect nurses at the bedside by focusing on components and education specifically designed for safer administration of chemotherapy. The fact that a multidisciplinary organisation is committed to supporting nursing safety speaks to the collaborative effort necessary in treating cancer patients, and provides a unified voice from several different countries. Unlike in the USA, where USP 800 has mandated closed systems for the administration of hazardous drugs, there is currently no equivalent requirement in Europe, despite impassioned pleas from leaders in nursing safety (Simons and Toland, 2015; Meade et al, 2017).
CSSA description
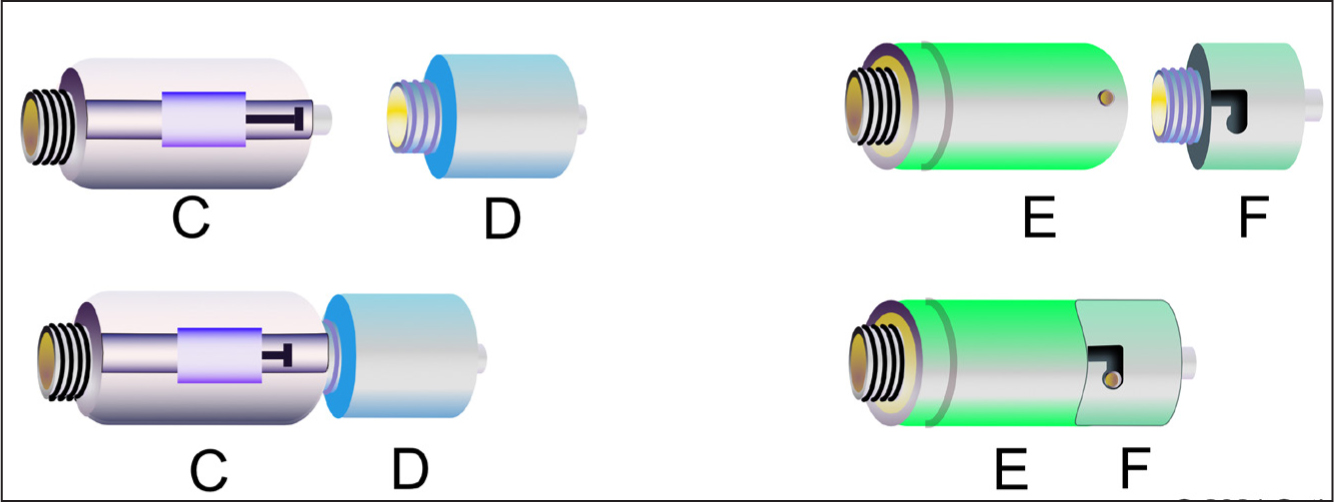
CytoPrevent defines a CSSA as a Luer format product that connects the distal end of an IV line (including elastomeric and electronic pump tubing), or syringe to a needleless connector to eliminate exposure. No preference is being given to any particular manufacturer. Table 1 provides a list of four common products currently available in Europe that meet this definition. CSSA designs are presented in Figure 2.
Table 1. Products meeting the CSSA definition
| Manufacturer | Product name |
|---|---|
| Baxter | Arisure / Clearlink |
| BD | Texium / Smartsite |
| ICU Medical | Spiros / ChemoClave |
| Vygon | Qimono (Qimomale / Qimofemale) |
A major point of emphasis with any CSSA is ease of use. Over the past two decades, the author has spoken to dozens of nurses in the USA who refused to use a CSTD despite these being provided by the healthcare organisation. This was due to CSTD design complexity, which made it difficult to use quickly at the bedside, the need for Luer adaptors, and equipment not being readily available. Another complaint was that once a Luer adaptor is connected to the IV tubing (for example, for an IV push), that injection port is no longer available for the administration of other non-hazardous drugs, such as antiemetics or analgesics. Adaptors can also hinder flushing of the patient's IV catheter since they would need to be removed to accommodate a standard Luer-based syringe of saline and/or heparin. One approach that some manufacturers have only recently started to embrace is designing IV tubing with both a Luer port and a built-in CSTD adaptor. Unfortunately, this increases the cost significantly and is limited to the specific brand of IV tubing and CSTD manufacturer as none of the membrane-based CSTDs are designed to be compatible with each other.
Luer-based designs are easy to use and do not require additional components. Their relative simplicity also incurs a lower cost when compared with non-Luer CSTDs. Cost of devices has been implicated as a major reason for lack of use historically in the USA and currently in other countries (Fazel et al, 2021). Considering the number one goal should be to protect the bedside nurse, the use of devices specifically designed for administration should be encouraged. Differentiating CSSAs from the more expensive and complex devices used for compounding can help achieve that goal while keeping costs to a minimum.
Conclusion
Clearly, much work remains to be done if we are to reduce nursing exposure. Education and easy-to-use products will not alleviate the problems without changes in safety culture. This culture, which is partially driven by varying regulations and practices in different countries, needs to focus on the primary goal of fostering nursing safety by working with device manufacturers and groups such as CytoPrevent. By coming together behind the CSSA concept, nursing educators and manufacturers can decrease exposure at the bedside. Leaders at both the national and hospital level must support initiatives to protect nurses who are providing care for cancer patients on a daily basis. Inconsistencies and ambiguities in guidelines, in combination with confusion in terminology have led to suboptimal safety. The fact that nurses are still continuing to suffer side effects associated with hazardous drug exposure is unconscionable given the overwhelming data amassed over the past 40 years and the availability of devices for preventing exposure. Considering the high number of cytotoxic agents given each day by nurses, we must look for innovative ways to educate and reinforce that education to keep our current generation of nurses—and future generations—safe from these important yet highly toxic drugs.
KEY POINTS
- Exposure to chemotherapy has been associated with a number of short-term and long-term side effects
- Nursing and pharmacy guidelines provide information on equipment that can prevent or minimise healthcare worker exposure
- The term ‘closed system’ can have different meanings for nurses, and this results in confusion in the literature and in practice
- Nurses need cost-effective and easy-to-use devices that prevent exposure during chemotherapy administration
CPD reflective questions
- Do you think you have received sufficient education and training for safe administration of chemotherapy/hazardous drugs?
- Are you concerned about potential exposure to chemotherapy?
- What types of safety improvements could be made to enhance safety for nurses?
- Have you experienced any of the exposure side effects discussed in this article?


