Wound management is a major clinical challenge and places a vast financial burden on the NHS (Guest et al, 2016) because of a rapid rise in long-term conditions such as diabetes, obesity and an ageing population (Lu et al, 2018). Damage to the skin impairs its functionality; this can include breaching of the physical barrier, which can reduce protection from ingress of pathogens, and affect temperature regulation and water loss. A physical breach of the skin provokes a wound-healing cascade of precisely synchronised events in five distinct phases: haemostasis; inflammation; migration; proliferation; and remodelling (Li et al, 2007).
The duration and character of the healing process indicate whether a wound is acute or chronic (Simões et al, 2018). Under normal physiological conditions, the restoration of the epidermal structure is highly effcient. An acute wound is one that heals in an appropriate manner, going through the phases of wound repair described above. However, a chronic wound is characterised by a defective healing process that does not allow skin to be repaired in an orderly and timely manner (Järbrink et al, 2016).
While wound healing is intrinsically affected by a variety of factors, a key challenge for clinicians is overcoming barriers to healing such as infection. Although bacteria are resident components of the intact skin microbiota, the expression of virulence determinants and the formation of biofilm (microbial communities) may impede wound healing (Wolcott et al, 2010). When the barrier function of the skin is impaired, bacteria can infiltrate into underlying tissues and this can lead to life-threatening infections (Negut et al, 2018). Despite recent progress made in wound management, wound infection remains a significant cause of mortality and increased morbidity (Negut et al, 2018).
In the initial stages of wound infection, Gram-positive organisms, e.g. Staphylococcus aureus, predominate. In the later stages, facultative anaerobic Gram-negative bacteria, such as Escherichia coli, Proteus species, pseudomonal species and Klebsiella, become more pronounced and tend to invade deeper layers of skin, causing significant tissue damage (Dow et al, 1999). If the immune system is not able to remove the pathogen(s), infection occurs and the expression of virulence causes tissue damage, compromising the normal wound healing process (Cooper, 2002). The formation of biofilms in open wounds is a cause for concern as microbes embedded in the associated slimy matrix have decreased sensitivity to antimicrobial agents (Cooper, 2002).
To ensure effective wound healing, it is essential to maintain a controlled set of conditions at the wound site (i.e. oxygenation, temperature and a high availability of vitamins, minerals, and trace elements) that sustain the complex cellular activity during this process.
Dressings have long been considered a critical component of wound care. They are used to promote a suitable environment for wound healing and protect damaged tissue from environmental and bacterial infiltration. Factors to consider in dressing selection include patient comfort, hygiene, duration of application, presence of local infection, ease of application/removal and avoidance of pain.
Ever-increasing evidence is demonstrating the effectiveness of medical-grade honey in the management of wounds through treatment of local infection, autolytic debridement, deodorising of wounds and promotion of tissue growth and granulation (Evans et al, 2013). It has broad-spectrum action and there have been no reported incidents of bacterial resistance to honey of significant importance (Cooper and Gray, 2012).
The main bactericidal substances found in honey are hydrogen peroxide (H2O2), bee defensin-1 and methylglyoxal (MGO). Together with these, the high sugar content in honey, which generates high osmolarity and the low pH, create a hostile environment for bacterial survival (Kwakman et al, 2008; 2010). Glycose oxidase is produced by the Apis mellifera species of honey bee, an enzyme that converts glucose into hydrogen peroxide and gluconic acid. Bee defensin-1, usually found in royal jelly, the major food source for queen bee larvae, is a cationic antimicrobial peptide that is effective against Gram-positive bacteria. Some studies using recombinant defensin-1 also report its activity against Gram-negative bacteria including Pseudomonas aeruginosa and Salmonella choleraesuis (Kwakman et al, 2011a; Bucekova et al, 2017). MGO is a 1,2-dicarbonyl compound that has been found to be the non-peroxide antibacterial constituent or unique manuka factor found in manuka honey (Atrott, 2009).
This product focus will describe how Revamil gel and Revamil gauze dressing (Bfactory Health Products, Rhenen, Netherlands) can assist clinicians to overcome wound care challenges.
Revamil honey dressings
Revamil wound dressing (polyacetate sheet dressing impregnated with pure honey) and Revamil gel, a hydrophilic wound gel consisting of 100% honey that is marketed as enzyme rich with a low pH of 3.5, are available in the UK. There are no apparent contraindications to their application, other than an allergy to honey or bee products, and they can be applied to a range of wound types.
Revamil products are sourced in the Netherlands from bee colonies under controlled circumstances. The controlled environment within which Revamil is produced helps to ensure that there is consistency in composition and therapeutic properties. As part of the production process, Revamil honey is sterilised by gamma radiation to kill any bacterial spores that may be present (Kwakman et al, 2011b).
Manuka honey is harvested through a more natural process from manuka trees and there is batch-to-batch variation in its antimicrobial properties (Kwakman et al, 2008).
Consequently, Revamil and manuka have different compositions of antibacterial components. Manuka honey has more than 44 times the concentration of MGO than Revamil. Bee defensin-1 and hydrogen peroxide, the major factors involved in rapid bactericidal activity of Revamil honey, are absent or found in very low concentration in manuka honey (Kwakman et al, 2008; 2010).
When the bactericidal activity of Revamil was compared to that of Manuka honey, Revamil was found to have a more rapid bactericidal effect, acting within 2 hours against species such as Bacillus subtilis, E. coli and Pseudomonas aeruginosa whereas manuka honey had such rapid action only against Bacillus subtilis. After 24 hours of incubation, however, both honeys killed all test bacteria, including methicillin-resistant Staph. aureus, with manuka honey being more potent over 24 hours. Manuka honey also retained its activity at higher dilutions than Revamil (Kwakman, 2011a).
Revamil honey has been shown to have potent in vitro bactericidal activity against multiple different antibiotic-resistant Gram-positive and Gram-negative bacteria. This medical-grade honey therefore has the potential to be used prophylactically or even as treatment for wounds infected with antibiotic-resistant bacteria (Kwakman et al, 2008).
Furthermore, studies have also shown that Revamil honey, when enriched with bactericidal peptide 2 (BP2), which is a synthetic peptide, has a rapid bactericidal activity against a range of species including MRSA and extended spectrum beta-lactamase (ESBL) E. coli at up to 10–20-fold dilutions. Revamil honey enriched with BP2 also had a broader spectrum of bactericidal activity than either BP2 or honey alone, so could have potential for further product development (Kwakman et al, 2011b).
Case studies
The following case series describe the management of a variety of wounds with Revamil gel and/or gauze dressings. In cases 1, 3 and 4 just gauze was used and in case 2 gauze and gel were used together. The four cases listed cover a wide variety of wound types and patient demographics and comorbidities. The treatment of each set of wounds was tailored to the individual patient over a variety of time periods.
As these dressings are established wound management treatments, no formal ethical approval was sought or required, but informed consent was obtained from all patients participating in this series along with consent to have their details and images used in this publication.
Case 1. Scalding in young child
A 7-month-old child sustained a scald to the left leg from an accidental spillage of hot water. The size of burn was estimated as be 1-2% of the total body surface area (TBSA). Infection is always a concern in small paediatric burns. Revamil dressings were applied, and the burn took 26 days to heal. The dressings were changed every 3–4 days, and the nurses said they were easy to apply and remove (Figures 1a and 1b). There was little exudate and the periwound skin remained intact. The cosmetic outcome was deemed very good.
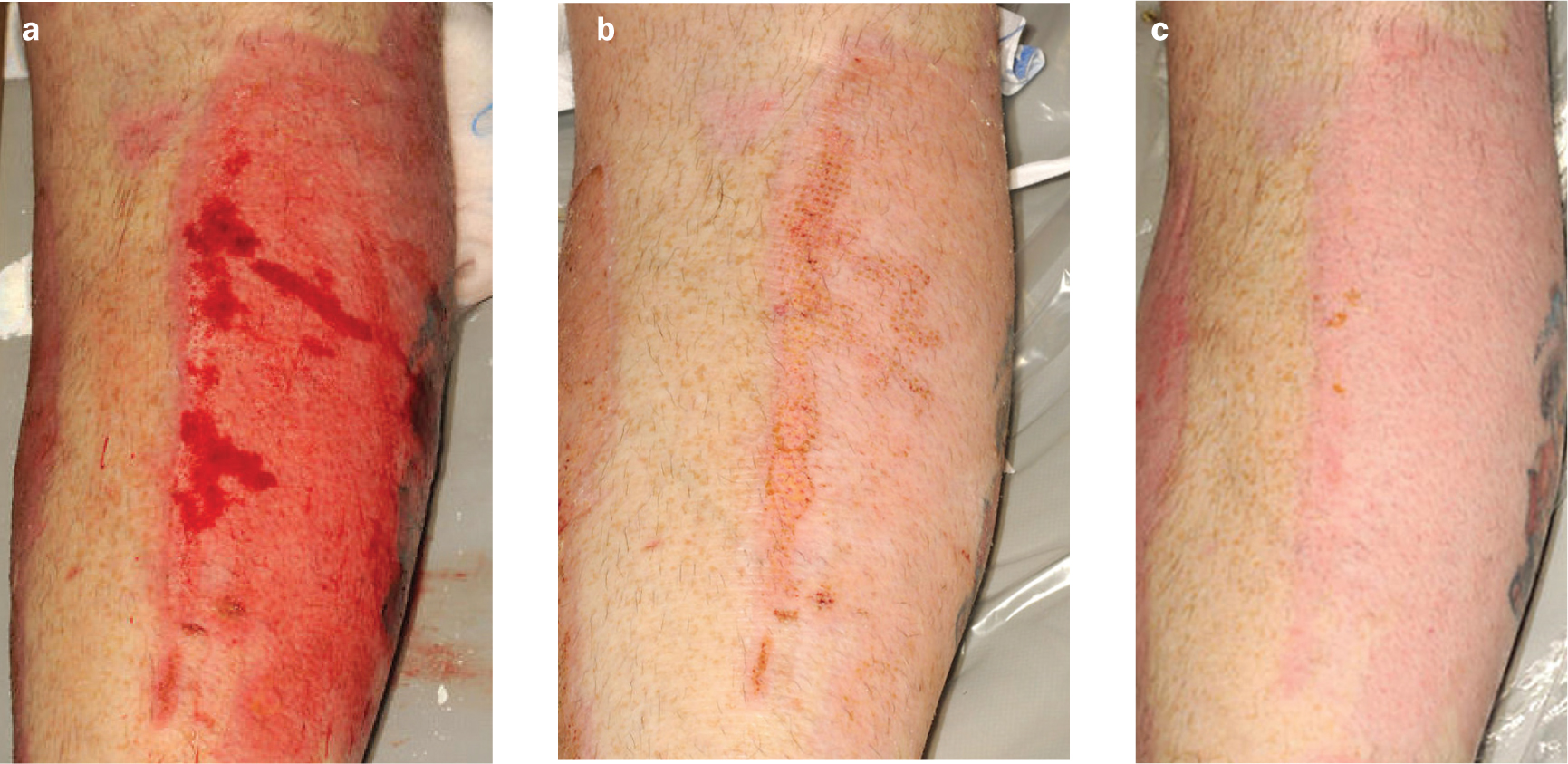
Case 2. Graft after burn injury
A 36-year-old man sustained flame burns which required grafting with split-thickness skin grafts. These were harvested from the outer aspect of the right calf. It is well recognised that silver dressings will impede the healing of split-thickness skin graft donor sites. Revamil was therefore used.
The dressings were changed every 3–4 days and came off easily, causing little pain. The donor sites healed completely within two weeks (Figures 2a, b and c), which is standard for the healing of a donor site. There was little exudate and the periwound skin remained intact. The cosmetic outcome was good.
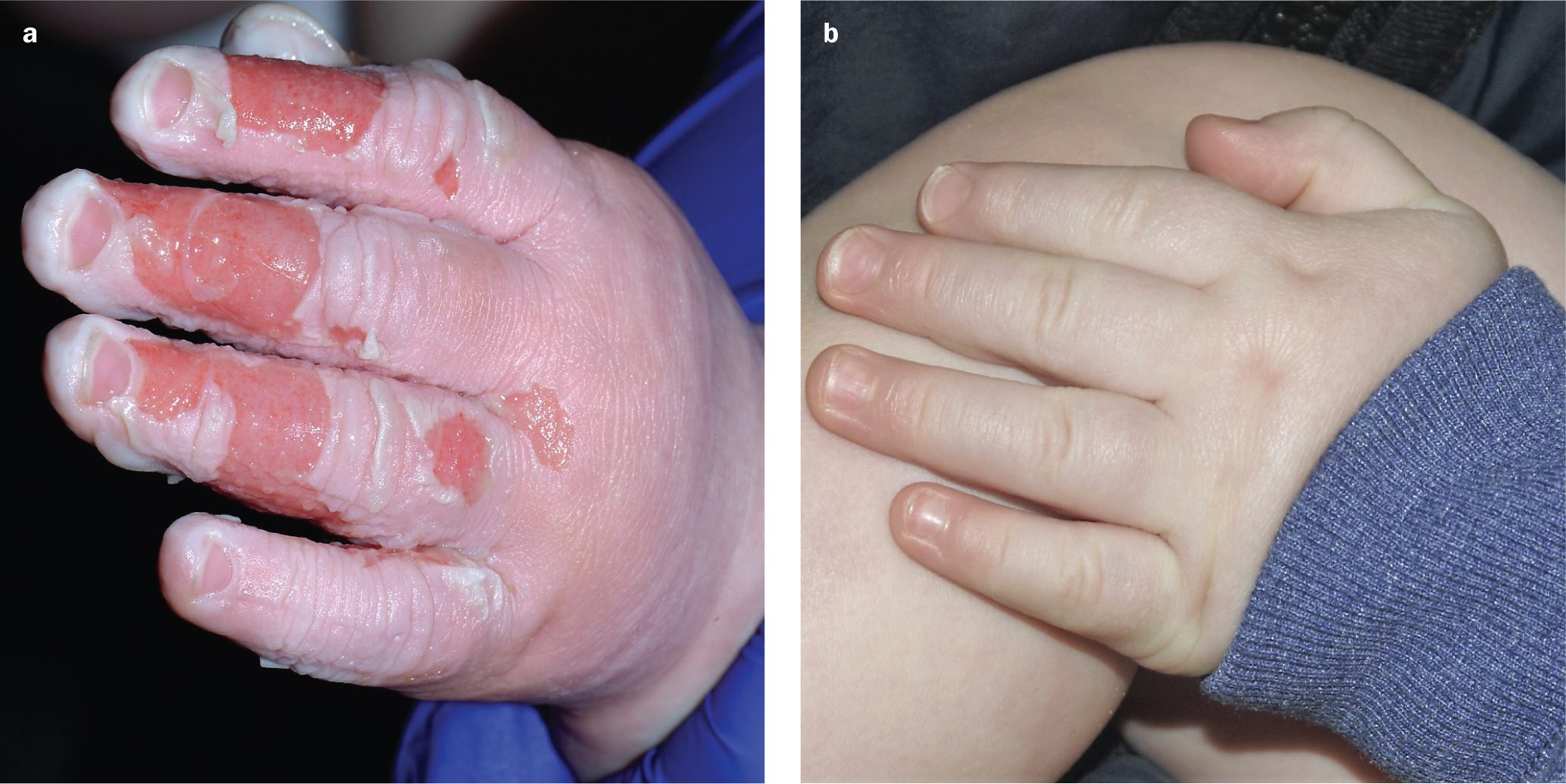
Case 3. Scalding in young child
An 8-month-old child presented with accidental scald burns to his left hand. He had accidentally placed his hand in a mug of very hot water. After providing first aid with cold water, the parents brought the child to hospital.
The burns were dressed with Revamil honey dressings and took 14 days to heal in total. The dressings were changed every 3–4 days and the nurses deemed them easy to apply and remove (Figures 3a and 3b). There was little wound exudate and the periwound skin remained intact.
Case 4. Toxic epidermal necrolysis
A 38-year-old woman was admitted to the burns centre having developed toxic epidermal necrolysis, which was thought to have been in response to the ingestion of paracetamol or ibuprofen. She developed loss of epithelium of 60% of her total body surface area, including the mucosal surfaces of the eyes, mouth and genitalia.
Her arms and abdomen were treated with 50:50 ointment, but dressings were required for her back so she could lie on it. Her back was dressed with Revamil gauze dressings, and healing took place within one week (Figures 4a and 4b). The dressings were easy to apply and remove.
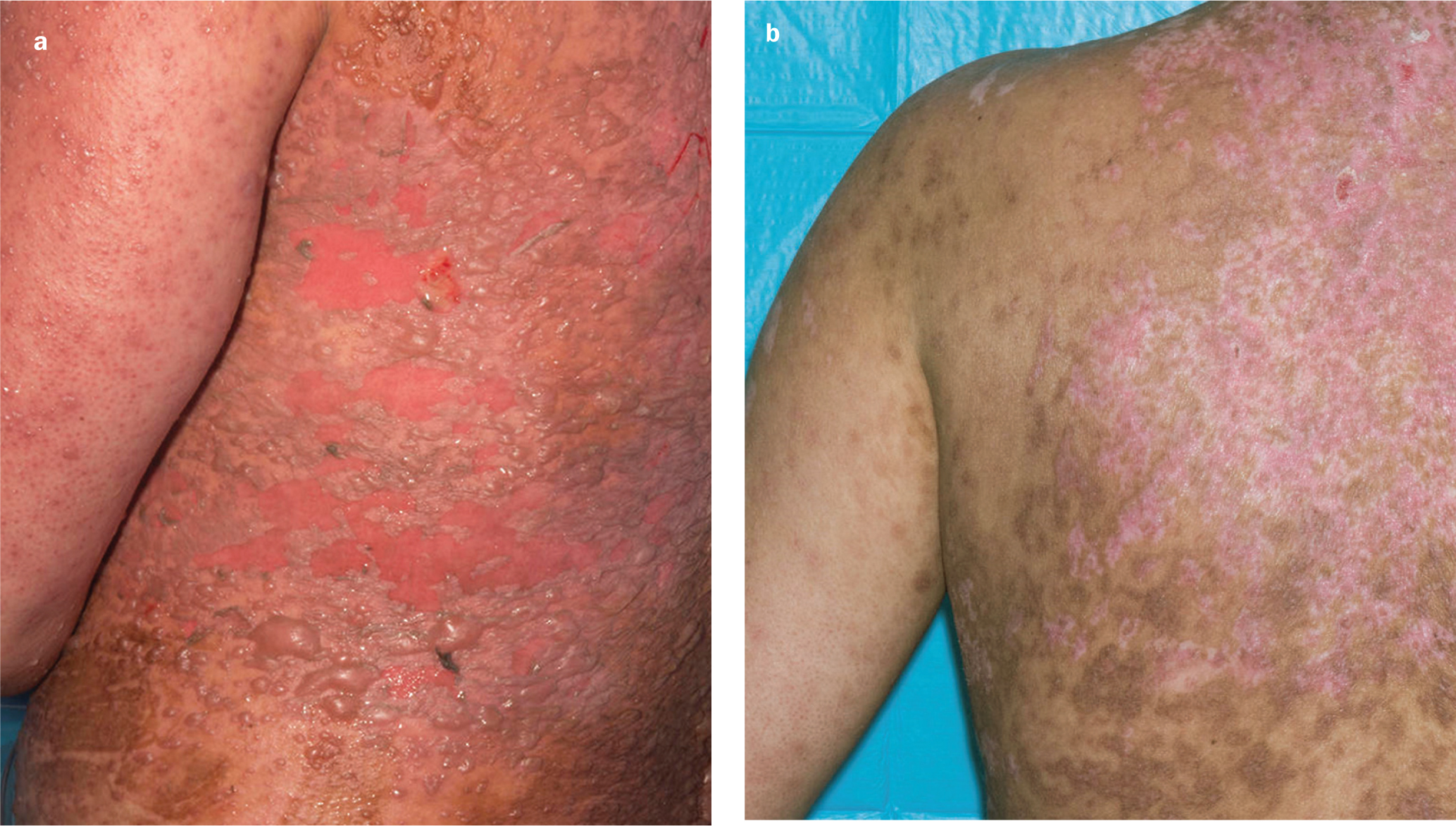
Revamil was chosen as the dressing because there was a theoretical risk of the patient developing a similar reaction to the sulphadiazine in silver sulfadiazine (SSD). The dressings were changed every 3–4 days and the nurses said they were easy to remove and apply. There was little wound exudate.
Discussion
A multitude of factors make choosing a suitable wound dressing a challenge. These can be patient-specific factors such as age, comorbidities, patient agreement and general wound healing propensity as well as other considerations, such as environmental factors, dressing characteristics and dressing cost-effectiveness. Therefore, each wound should be managed on an individual basis.
Medical-grade honey dressings in general have been shown to be advantageous in their ability to promote autolytic debridement of devitalised tissue within wounds, significantly reducing malodour and pain. Overall, honey as an agent for autolytic debridement has been considered effective in wounds that contain ^40% devitalised tissue (Evans and Mahoney, 2013). This factor is particularly important when dressing chronic, sloughy or overgranulating wounds.
As shown in this series, Revamil gauze and gel can be used to treat a variety of wounds and patient indications. The dressing creates a moist wound environment, which stimulates healing, neutralises wound odours and creates a protective layer over the wound (Oswell Penda Pharmaceuticals, 2019). With few or no contraindications, honey dressings have significant advantages over other antimicrobial dressings such as SSD.
In a retrospective review of 108 patients with burns, honey dressings achieved wound sterility in 7 days while there were no recordings of sterility with SSD-treated wounds. The average times to healing were 18.16 days and 32.68 days for the honey and SSD group respectively. A complete recovery was seen in 81% of all patients treated with honey, while only 37% patients in the SSD group showed complete recovery. Honey dressings also had better outcomes in terms of hypertrophic scars and post-burn contractures than SSD dressings (Gupta et al, 2011).
One of the main benefits of Revamil gel and gauze is comfort for patients during application and removal; this is of particular importance in paediatric patients. Revamil gauze dressing does not stick to wounds, so will not compromise the underlying wound bed nor cause pain on removal. It was noted that all Revamil dressings applied to paediatric patients in this series were applied and removed with ease, causing minimal discomfort.
Conclusion
It has been claimed that honey dressings have been shown to be a reliable and cost-effective choice, particularly in developing countries (Moghazy et al, 2010). They also offer a viable alternative to silver-based dressings.
Revamil honey dressings can be used to treat a variety of wounds and patient indications. The dressing provides a moist wound environment, stimulates healing, neutralises wound odours and provides topical protection.

