Every day the human body is exposed to a huge number of viruses. Although most are harmless, some, which are known as pathogenic virions, can cause disease. Virus particles come in various shapes and sizes and the individual characteristics, together with the characteristics of the individuals they infect, impact on their virulence, including their mode of spread. They also impact on the ability of the immune system to mount an effective and sustained response to prevent disease. When this is not possible, development of a robust, safe, and long-lasting vaccines is sought (Doms, 2016).
On 31 December 2019, a new coronavirus (SARS-CoV-2) was identified from ‘a cluster of pneumonia cases of unknown aetiology with a common source of exposure’. By 10 January 2020, its genome sequence was released to the world (Zhou et al, 2020). By the end of the same month, its emergence had been declared a Public Health Emergency of International Concern. On 7 February, the disease it causes was named COVID-19 (Coronavirus Infectious Disease 2019), and within 6 weeks had been declared a pandemic (European Centre for Disease Prevention and Control, 2020). It would be great to say that the rest is history but sadly it is not.
As of 18 August the number of reported deaths and number of new cases continues to rise. Globally, at the date of publication, there are 26 198 228 cases of COVID-19 and 867 755 deaths, with 215 countries and territories affected (Worldometer, 2020). The way in which deaths attributed to COVID-19 are calculated in the UK has recently changed. As of 11 August the death rate includes only people who had a positive test within the 28 days preceding their death, resulting in a drop of around 5000 in the reported figures. Nevertheless, to date the UK has recorded 41 514 deaths; this is the highest total number of deaths per country in Europe and the fifth highest death rate in the world (Worldometer, 2020). The race is on for a vaccine. The speed at which a vaccine can be produced is clearly of vital concern to everyone, but especially to nurses who continue to advise the public on prevention and to deliver care of patients affected by COVID-19. Of further interest, however, is an understanding of the types of vaccine in development and the implications for delivery by nurses who will again be at the front line of delivery.
What are coronaviruses?
Micro-organisms are classified into groups (microbes) according to their key characteristics. These include viruses, bacteria, protozoa, helminths, fungi, prions and ectoparasites. Microbes that have the potential to cause disease are called pathogens and the largest category by far, in terms of numbers identified, are viruses. Viruses are extremely small organisms, so much so that they cannot be viewed under a standard microscope. They exist in independent form as viral particles, or virions, and require a host organism to replicate. Their central core contains genetic coding in the form of DNA or RNA. This is referred to as the genome. The unique attributes of the viral particles determine their ability to enter host cells and to replicate their viral genome.
Coronaviruses were first identified more than 50 years ago (Almeida et al, 1968). Around 40 varieties have since been identified, seven of which are now known to transfer to humans from non-human mammals and birds (Aronson, 2020). The first four types of coronavirus tend to cause mild respiratory symptoms although, like most illnesses, severity is greater among immunocompromised individuals. The fifth coronavirus, SARS (severe acute respiratory syndrome), and sixth, MERS (Middle Eastern respiratory syndrome), were far more serious (Cui et al, 2019). The seventh, which is COVID-19, is thought to have originated in bats, subsequently spreading to snakes and pangolins, and infecting humans through the ingestion of contaminated meat from wild animals (Aronson, 2020).
The genome of SARS-CoV-2 is a single thread of RNA. This is significant, in that it makes the virus more prone to mutation. The viral genome is protected by an outer coating, which may be single layer (non-enveloped) or a more complex double layer (enveloped)—SARS-CoV-2 is enveloped. Its genetic core is protected by a fatty outer layer, which aids infiltration of host cells and helps them evade the body's usual immunological response (Figure 1). Several different proteins surround and emerge from this lipid layer, each with a unique role to play. Of particular interest are the ‘spike’ (S) glycoproteins, which are arranged in groups of three on the particle surface giving the virus is characteristic appearance (Figure 1). The S protein facilitates attachment to host cells and, as will shortly be discussed, is central to the focus of several vaccines currently in advanced stages of clinical trials (UK Research and Innovation (UKRI), 2020).
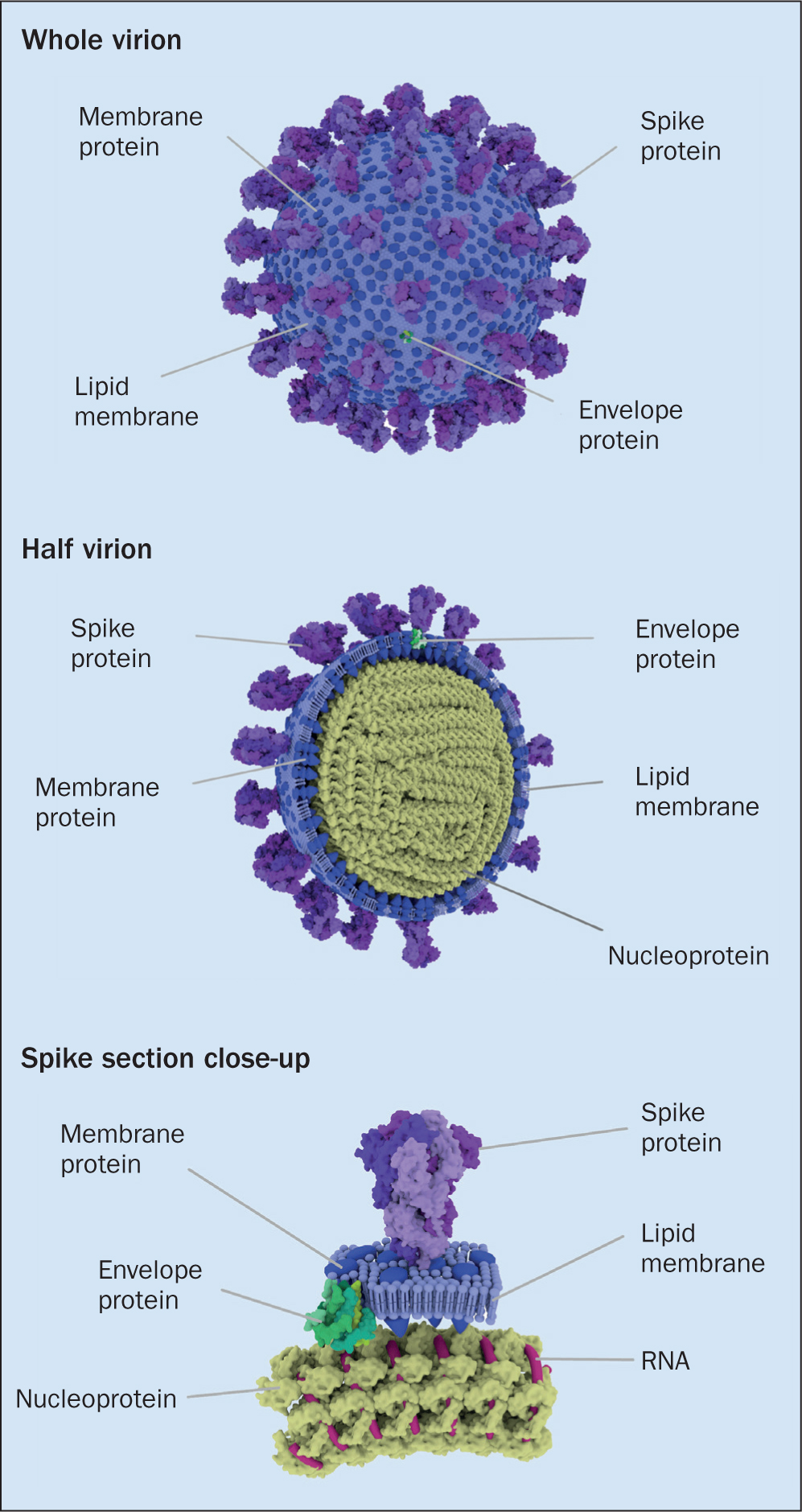
How does SARS-CoV-2 attempt to cause disease?
A ‘whistlestop’ revision of immunology
Medawar (1977) classically described viruses as ‘bad news wrapped up in protein’. The aim of a vaccine is to stop this ‘bad news reaching those for whom it may be devastating’. In order to understand how vaccines have traditionally worked and the new approaches under trial, as well as the implications for nurses who will ultimately deliver vaccines to patients, it is necessary to review some basic immunology.
The purpose of SARS-CoV-2's unique design is to ensure that the virus can attach to the surface of uninfected host cells, penetrate the cellular membrane and replicate its genome before escaping into the recipient's bloodstream with the possibility of causing widespread disease. The number of viral particles needed for the virus to cause disease (viral load) differs according to sub-species. Given the infectivity rate of SARS-CoV-2 it is likely to be relatively low (McIntosh, 2020). In addition to the structure and genus of the microorganism itself, the virulence and ability to prevent specific disease is influenced by the vector (carrier organism) characteristics of the host and the ability to impact upon the mode of spread. These characteristics, particularly the latter, vary in different contexts and environments (McIntosh, 2020; UKRI, 2020).
A well-functioning immune system can potentially block this process through a range of highly complex processes. These are finely tuned and mediated by specific cells. Specific parts of pathogens, known as antigens, can be recognised as ‘non-self’ by the immune system, alerting it to the threat of infection. There is a whole host of mechanisms involved in this process and the threat can be blocked at various stages by the complex mechanisms of the immune system. When viral particles enter the circulatory system, the immune system attempts to prevent them entering host cells by inactivating their viral proteins and stimulating the production of lymphocytes. These lymphocytes can be subclassified according to their function. The recognition of specific antigens in the viral particle by B cells (orchestrated by T cells) and the subsequent multiplication of antibodies specific to that antigen are central to preventing widespread disease and leave an imprint known as immunological memory. This means that subsequent infection by the same virions is recognised and a quick response is mounted.
There are dangers with both a poor response and an over-response. Although antibodies are needed in abundance to ‘fight’ the virus, T-suppressor cells have an essential role in regulating the quality and level of the immune response (Public Health England (PHE), 2018). This is a delicate balance because excessive stimulation causes generalised inflammation, which can result in organ shut down the so called ‘cytokine storm’ (Coperchini et al, 2020).
Why is a vaccine needed?
It is apparent that the immune response in some individuals is insufficient to protect against SARS-CoV-2, causing COVID-19 and potentially death. Furthermore, the strength and length of immunological memory in those who successfully develop antibodies from acquiring the disease is under question. An early preview from a study at King's College London has found that antibodies in COVID-19 patients vanished 2 months post-infection (Seow et al, 2020). If proven, this is likely to be challenging in terms of developing a robust vaccine. Research to establish whether this is the case is ongoing.
The aim of vaccination is to stimulate the body's immune system into a response similar to that caused by invasion by the targeted pathogen, but without developing the illness. Immunological memory is essential in affording ongoing immunity to future infection. Importantly, a robust vaccine with a good uptake rate also halts the spread of the virus to other people (UKRI, 2020). In the immediate term, efforts are also being focused on the possibility of passive immunity from SARS-CoV-2, using antibodies that have already been created by the immune system of donors (Eli Lilly & Company Press Release, 2020). This form of passive immunity is already used to give short-term protection against other antigens, such as hepatitis B and rabies.
What does the race involve?
‘The race to produce a COVID-19 vaccine is multifaceted—involving new research on many levels, enormous amounts of fundraising, and unprecedented international cooperation.’
Understanding the characteristics and pathogenesis of SARS-CoV-2 is vital in terms of vaccine development, but it is only the beginning. This knowledge must then be applied to determine which parts of the immune system need to be stimulated or challenged in order to ensure a robust, long-lasting and, above all, safe vaccine-mediated immune response (Morrison and Plotkin, 2016). The completion of human genome mapping, along with recent progress in DNA sequencing (including that of pathogens), has given rise to a whole host of new possibilities in terms of vaccine technology. To date, these have been used successfully for some pathogens, such as meningitis B (Boulton, 2013).
Although attempts to develop a vaccine for earlier coronavirus outbreaks have been unsuccessful, much useful knowledge has been gained and is being applied to current attempts to develop a vaccine to protect against COVID-19 (Caddy, 2020). The publication of the genetic sequence of SARS-CoV-2 on 10 January 2020 (Zhou et al, 2020) was the starter's gun for the current race to develop a vaccine. At the time of writing, there are 210 vaccines in development, 30 of which have entered clinical trials (Milken Institute, 2020b) (Figure 2).
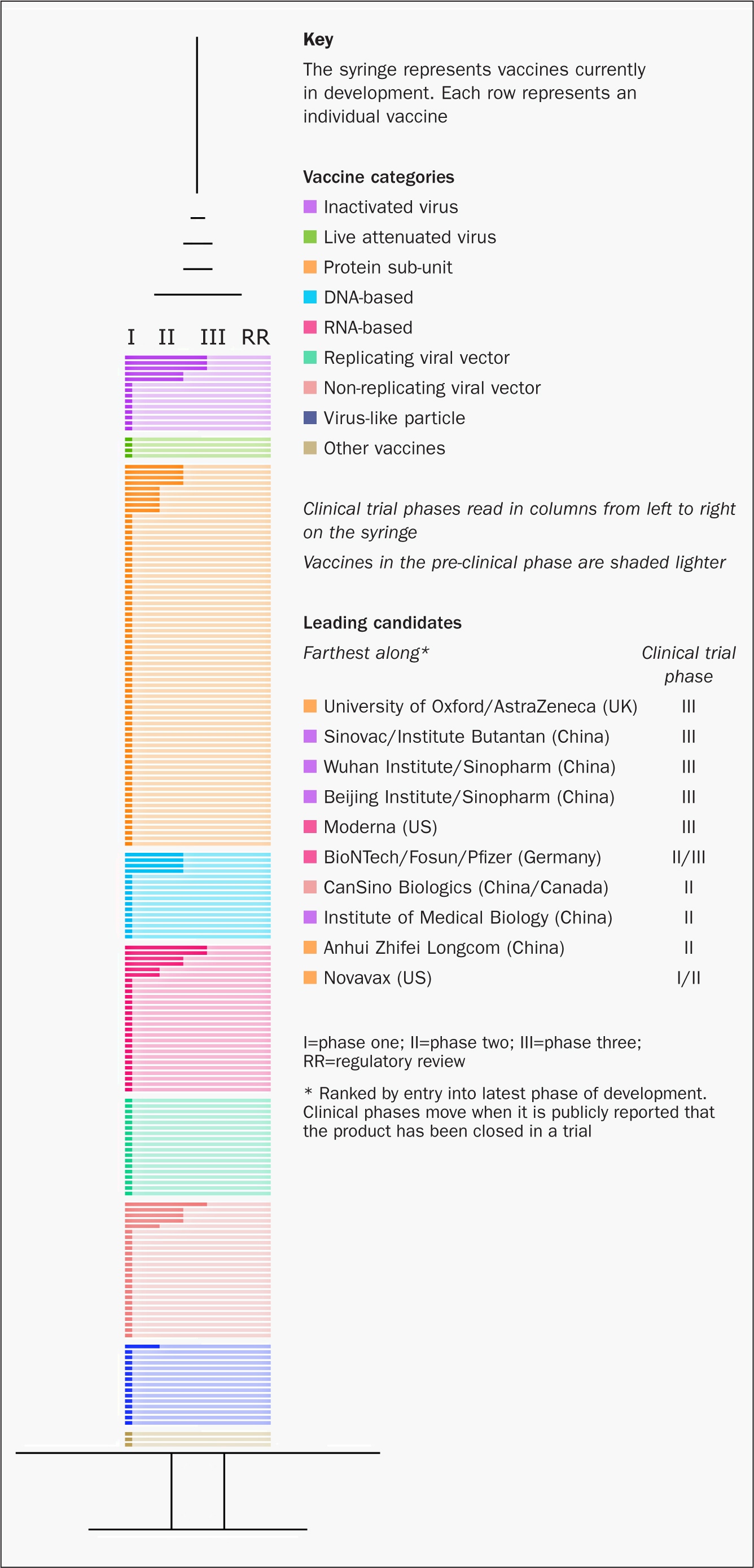
The runners and their attributes
Before a vaccine can be licensed for widespread use a robust and carefully regulated process is followed. This includes registration and publication of results at each of the three stages of clinical trials to establish safety and efficacy. Historically, the process of developing a vaccine is lengthy, taking on average around 10 years from conceptual phase to licensure. The recent Ebola outbreak saw a vaccine produced in record-breaking time. Today, the ongoing global threat of COVID-19 has accelerated the race for a viable vaccine. Russia has recently developed and approved its own COVID-19 vaccine for widespread use, despite it apparently undergoing only a phase I trial with 38 people (Mahase, 2020). This is only likely to feed public concerns in the UK and elsewhere about vaccine safety.
It is therefore vital that nurses understand the testing process (Table 1), together with the new approaches to vaccine development that offer the possibility of hugely accelerated time scales, including the amalgamation of trial phases (Kim, et al, 2020). Vaccine companies and research institutes have also teamed up at early stages so that vaccine production can keep pace with research, rather than waiting for potential vaccines to be licensed before production commences. Several organisations including the World Health Organization (WHO), have produced vaccine trackers, but the one produced by the Milken Institute (2020b) is particularly user friendly (Figure 2).
| Stage | What happens | Why | Usual timescale | COVID timescale |
|---|---|---|---|---|
| 1. Exploratory phase | Studies into microbe particles thought to produce disease are carried out in laboratories and usually involve animals | Understanding these processes is necessary prior to exploring the prevention of disease caused by the pathogens | ||
| 2. Application to begin testing on human subjects | Formulate and submit detailed protocol to undertake research in humans. In the UK, this is via the Medicines and Healthcare products Regulatory Agency (MHRA) | Due diligence in terms of complying with international and national legal frameworks, ethical considerations and suitable funding must be shown | 6–12 months | Accelerated efforts for quick review. Oxford vaccine reviewed and authorised in little over 1 working week |
| 3. If approved, the study formally enters preclinical trials and is recorded on a database | ||||
| 4. Clinical trials | ||||
| Phase I | Randomised, double-blind, controlled small-scale study in 20–100 healthy volunteers | Mainly looking at safety and immune response at different doses |
1–2-years | Three months |
| Phase II | Randomised, double-blind, controlled study of hundreds of people that further evaluates safety, but is particularly focused on efficacy | More concerned with strength of immune response |
||
| Phase III | Randomised, double-blind, placebo-controlled study of thousands of people | Evaluates both safety and efficacy in intended recipients | ||
| Licensing | Review of licencing application by government bodies (currently, the MHRA in UK) | 1–2 years | A few months | |
| Phase IV | Post-approval studies monitoring effectiveness | Safety of vaccines in the UK and European Union continues to be monitored. The addition of a ‘black triangle’ indicates ongoing intensive pharmacovigilance | Minimum 6 months | Likely to be more than 6 months |
In terms of progress, the contenders tend to be assessed according to the phase and size of vaccine trials. However, the technological approach will also have a bearing on the attributes, efficacy, cost-benefits analysis, speed of production and possibly delivery mechanisms of successful vaccines. As such, understanding them and the reasons why the process of testing can be so accelerated of is of great importance to nurses who will be at the front line, answering patients' concerns and delivering the vaccine. The Milken tracker groups the candidates into 9 categories according to the technological approach used (if the 9th category of nonhomogeneous approaches is included). As can be seen in Figure 2, of the current top five, although three are inactivated vaccines, the other two are based on non-traditional approaches. Of the top five candidates, in terms of progress, three are based in China, one in the UK and one in the US.
Traditionally, approaches to vaccine development have involved modifying the viral particle itself (in entirety or partially), or using one of its secreted products, eg tetanus toxoid (PHE, 2018). This is the approach taken by the three Chinese research institutes that are in the top five. However, top of the leader board at present is the Oxford-based Jenner Institute's non-replicating viral vaccine. This same technology is also being adopted by the San Casino Institute in China.
The Jenner Institute uses a modified adenovirus (named from tonsoid tissue in which it was first isolated), which is found in chimpanzees but does not cause disease in humans. Adenoviruses are already being used for other gene-based therapies and are also the focus of attempts to develop other novel vaccines by the Jenner Institute, thus giving it a head start. The central core of this virus is double-stranded DNA, rather than the single-stranded RNA of SARS-CoV-2, meaning that the virus is less prone to mutations. The genetic code of the SARS-CoV-2 spike protein is added to the modified adenovirus and carried into the body's cells. These cells then produce S protein, evoking a full immune response. The adenovirus itself is non-replicating, which means that it cannot stay in the body.
A randomised controlled trial for this vaccine using MenACWY vaccine as a control started on 23 April (Folegatti et al, 2020). The vaccine is now at stage III, with the results of phase I/II trials showing robust immunogenicity in terms of activation of both antibodies and T cells and no early safety concerns (UKRI, 2020). It is postulated that this approach will elicit a much stronger overall response in the immune system than other technologies in the running. For nurses, this means that the schedule should require fewer doses and will be suitable for a wider range of age groups. As already mentioned, the knowledge gained in attempts to develop vaccines against other pathogens using this approach should make progress quicker than for some of the other contenders.
The approach favoured by the three Chinese institutes currently in second, third and fourth place is more traditional. Many vaccines fall into this category, including those for polio and hepatitis A. All vaccines in the top five, including these three, have now entered phase III trials (Clinical Trials Arena, 2020), but detailed information is more difficult to obtain. All three Chinese institutes are using a more traditional technology, focusing on vaccines containing the virion particle, or sub-particle in an inactivated form. This means that the virus is grown in laboratories (usually with egg culture) and then ‘killed’ to reduce its ability to cause harm. This is particularly important in ensuring that the vaccine is suitable for those who are immunosuppressed.
Vaccines are subdivided according to the method used to deactivate them. This depends on whether the viral component required for the vaccine will be the whole cell or a specific part. In the case of the vaccine against COVID-19, this appears to be unknown. These vaccines usually require several additional additives to deactivate and preserve the antigen. Since the immune response also tends to be less strong than live attenuated vaccines an adjuvant is also usually required (PHE, 2018). From the nurse's perspective important considerations are likely to be the risk of allergic reactions to any of these components.
The vaccine currently in fifth place is funded by the US and incorporates a new technological approach based on an mRNA. The same approach is being used by the vaccine under phase I and II trials at Imperial College London. The principle was first developed around a decade ago but, as yet, no RNA vaccines have been approved for use in humans. It involves introducing genetic material in the form of manipulated RNA code from SARS-CoV-2 into the host. This instructs host cells to make the spike protein in situ and to carry this forward onto the surface of the cell, where it can be recognised as ‘foreign’ by the host's immune system. An antibody response specific to the spike protein should then be generated along with a memory for this antigen. If the vaccinated individual is subsequently exposed to SARS-CoV-2, the immune system should respond rapidly by producing multiple antibodies in a short time period (UKRI, 2020).
This approach has the potential to be developed much more quickly and easily than other vaccines (Kim et al, 2020). Importantly, in terms of cold-chain issues, it would be more stable over higher temperature ranges, with the possibility that it could even be stored at room temperature for up to 18 months. This is particularly encouraging for a roll-out in lower income countries. It is also claimed that these vaccines will be the least expensive to produce and are better geared toward triggering a T-cell response. However, this could be at the expense of poor antibody development or waning of long-lasting immunity. At present, studies show that this vaccine is less effective in people aged 55 years and older, a key target for COVID-19 vaccination (Hotez et al, 2020; Zhu et al, 2020)
Of the other four approaches being trialled, it is worth noting that attempts to produce live attenuated vaccines are the least popular, probably due to the virulence of the virus. Other vaccine strategies under consideration include injecting DNA coding for the spike protein or injecting the actual spike protein in whole or in part (‘recombinant protein’).
Conclusion
The arrival of the novel SARS-CoV-2 virus has fixated the world on the search for a vaccine.
Although tremendous strides continue, it is clear that the implications for practice will depend on which approaches are finally licensed. Ultimately, the uptake of any potential vaccine will also depend on the public's perceptions of immunisation and risk. Currently, vaccine hesitancy is one of the top 10 global threats and attitudes to a COVID-19 vaccine remain to be seen. As discussed above, this, together with the specific attributes of any vaccine in terms of immunogenicity, storage, cost, schedule delivery systems and side-effects, all have implications, particularly for nurses who are at the front line addressing patients' concerns prior to its delivery.

