Venous access is a taken-for-granted aspect of contemporary disease management, yet for many people with complex health needs, obesity, diabetes, and/or a history of injecting drug use (IDU), venous access is time consuming and painful.1,2,3 Difficult venous access (DVA) is characterized by patient distress,2,4 veins that are difficult to visualize and palpate,5 and in our service, is defined by either 2 failed venepuncture attempts on a single occasion or multiple attempts on previous occasions.
As advanced practice nurses working in a tertiary gastroenterology/liver center, we identified DVA as a significant clinical problem. An earlier survey reported that over one-third of our liver outpatients experienced DVA, nearly one-quarter were reluctant to have venepuncture, and 12% felt bad or discriminated against. Of concern, 12% believed DVA prevented their access to therapy. Figures were worse for our drug health service, where 59% of respondents described DVA, 66% were reluctant to have venepuncture, and 36% felt bad or discriminated against.6
Many liver clinic patients have hepatitis C, with a history of IDU. People with IDU have a high prevalence of DVA (65–100%),7,8 and this combination is a known impediment to seeking and receiving health care.2,8 Overall, DVA causes frustration for staff,9 distress for patients, and suboptimal care access.6
Some techniques may improve venepuncture success, such as lessening the insertion angle for older patients, compressing veins to displace fat tissue in obese patients, and anchoring rolling vessels by either pulling the skin taut or by arm repositioning.10 Active limb warming,4 seeking wider veins (>0.4 cm) at moderate depth,11 and clinical evaluation of DVA status prior to cannulation can significantly improve success.3
For decades, ultrasound (US) guidance has been the most common method for managing DVA. Two systematic reviews and meta-analyses examined the benefits of US-guided procedures.12,13 While US guidance was more likely to lead to successful cannulation, neither review found evidence of a reduction in skin punctures or time to cannulation. A third systematic review identified significant variation in the definition of success between studies, concluding that routine US guidance was not well supported.14
Despite the early work of ours and others, few studies report the patient experience of DVA. Available literature has focused on venepuncture access for patients with IDU and DVA in the setting of hepatitis C treatment. One descriptive, qualitative study described the experiences of 10 people with hepatitis C, IDU, and DVA requiring venepuncture.15 Participants identified cultural, psychological, and organizational conflicts. Their emotions included guilt, shame, stigma, fear, and feeling like a ‘pin cushion.’ Participants described missed opportunities to engage them as experts and suggested concrete solutions to ameliorate DVA through enhanced communication and collaboration. 15 In a qualitative study exploring self-injection and harm reduction, participants felt judged by clinicians when seeking advice about their DVA.16
A key objective of venepuncture for the purpose of blood collection is to succeed on the first attempt with the least possible stress and pain. One innovative strategy, external jugular venepuncture (EJV), was previously implemented in a group of DVA patients attending the study setting.6 This strategy involved insertion of a winged 21G butterfly needle into the external jugular vein for the sole purpose of blood collection. The butterfly needle was removed immediately after venepuncture.
Potential risks of this approach were considered, in particular air embolism. A review of the literature revealed only isolated case reports of air embolism associated with insertion or removal of an indwelling external jugular central venous catheter17 or an uncapped hub of a cannula,18 rather than blood collection with a small gauge needle. Air entrainment is thought to be dependent on the volume of air that enters the bloodstream,19,20 and the lethal dose for humans has been theorized at 3–5 mL/kg,20 suggesting that a brief blood collection procedure by a trained operator with a small gauge needle is safe. Nevertheless, to minimize embolism risk, the EJV blood collection was undertaken with the patient in a supine position.20 Universal precautions were maintained to reduce the risk of blood-borne infection.
An EJV protocol and education package were developed collaboratively between the liver and anesthetics teams. Three master's-prepared hepatology advanced practice nurses (2 clinical nurse consultants and 1 nurse practitioner) undertook these EJV procedures. They had significant clinical expertise in venepuncture and undertook additional training and accreditation in EJV blood collection, consistent with the service's protocol. EJV is now considered a highly effective, usual practice within our clinical service. An audit of occasions of blood collection via EJV showed high patient satisfaction and no clinical complications.
While an intervention may be deemed effective in reaching a clinical goal, this may not extend to acceptability for the person receiving care. Acceptability of health care interventions is defined by several authors, and the major tenets include congruence with lifestyle, convenience, and effectiveness in addressing clinical issues.21,22 Sidani et al. added a temporal element, noting the importance of acceptability prior to engaging with an intervention.22 Andrykowski and Manne23 extended acceptability as a dynamic concept that may change over the course of treatment. Finally, Sekhon et al.,24 whose framework provided our theoretical lens, refined the definition to:
‘A multi-faceted construct that reflects the extent to which people delivering or receiving a healthcare intervention consider it to be appropriate, based on anticipated or experiential cognitive and emotional responses.’
Given the importance of acceptability to patient engagement, our study aimed to explore both the experience of DVA and peripheral venepuncture for liver patients and the subsequent experience and acceptability of EJV to inform recommendations for practice. A secondary aim was to explore the impact of EJV on local resource utilization.
Methods
Design
This descriptive, qualitative study25 used in-depth interview data to further understand DVA and EJV. The acceptability framework of Sekhon et al.24 provided an analytic lens. Supplemental quantitative audit data provided additional information about local resource utilization. The setting was a single outpatient liver clinic in a publicly funded teaching hospital in Sydney, Australia.
Qualitative arm
Sample: Purposive sampling identified people with DVA attending the study setting for liver disease surveillance who had experienced EJV. Potential participants were excluded if <18 years of age, if unable to speak English fluently, or if unable to provide informed consent. Potential participants were approached by a researcher (who did not provide them direct clinical care) to explain the study and provide a participant information statement and consent form. Where patients had left the clinic, recruitment documents were posted with covering letter and prepaid, return addressed envelope. Potential participants were invited to return the consent form or to contact the researchers with any questions.
Sample size was guided by the richness (a multilayered quality) and thickness (quantity) of data.26 Recruitment continued until major themes were richly developed, including negative case examples.27 The final sample comprised 10 patients (7 males, 3 females). While female gender is a risk factor for DVA,1 the higher ratio of males in our sample is typical of the broader liver disease cohort.28 Characteristics of participants appear in Table 1.
| Pseudonym | Age range (years) | Gender | Aetiology | DVA (years) | EJV (years) | History of IDU |
|---|---|---|---|---|---|---|
| Bill | 30–39 | M | Biliary atresia | >5 | 2 | No |
| Mac | 50–59 | F | Hepatitis C | 20–30 | 5 | Yes |
| Steve | 50–59 | M | Hepatitis C | 10 | 7 | Yes |
| David | 50–59 | M | Hepatitis C | 20 | >10 | Yes |
| Guy | 60–69 | M | Hepatitis C | 20 | 5 | Yes |
| John | 50–59 | M | Hepatitis C | 20–30 | >10 | Yes |
| Jan | 50–59 | F | Hepatitis C | >10 | 3 | Yes |
| Anne | 60–69 | F | Biliary disease | 20–30 | 5 | No |
| Rick | 60–69 | M | Alcohol and hepatitis C | 30 | 9 | Yes |
| Matt | 50–59 | M | Hepatitis C | 20–30 | >10 | Yes |
DVA=difficult venous access; EJV-external jugular venepuncture; IDU=injecting drug use
Data collection: Single, face-to-face semistructured interviews were conducted in a private clinic room using an interview guide. This usually occurred during a clinic visit by a researcher not providing direct clinical care (SM, SS). Interviews (duration 18–46 minutes) were audio-recorded, professionally transcribed, and were supported by detailed field notes.
Data analysis: Initial data were inductively coded separately by 2 researchers (SM, SS), then discussed to achieve consensus with a third researcher (JG). We then used the acceptability framework of Sekhon et al.24 as a further theoretical lens to organize these themes under temporal, experiential, cognitive, and emotional responses before and after patients’ engagement with EJV (Figure 1).
Quantitative arm
Sample and Data Collection: Audit data were collected from experienced venepuncture nurses servicing a liver clinic from 24 occasions of service (71% EJV and 29% peripheral venepuncture). As consent was implied by survey completion and data anonymously provided, the characteristics and final number of nurse participants providing survey data cannot be described. Data included number of venepuncture attempts, venepuncture success, assistance required, position, access site(s), and procedure time, with opportunity for short qualitative responses.
Ethical considerations
The Sydney Local Health District Human Research Ethics Committee (Ref: HREC/15/CRGH/251) approved the study protocol, which was developed using Australia's National Statement on Ethical Conduct in Human Research.29
Qualitative arm: Written, informed consent was obtained. Interview data were de-identified; participants are referred to by pseudonym. A participant distress protocol was available in case of distress during interview. While we made provision for access to counseling, none was required.
Quantitative arm: Anonymous nurse survey data were returned to a sealed box. A researcher who did not work with venepuncture nurses (and so did not recognize their handwriting) completed data entry to maintain confidentiality.
Rigour
The qualitative study was designed, conducted, and reported using the Consolidated Criteria for Reporting Qualitative Research checklist.30 An audit trail of research-related decision making was maintained. Multiple data sources31 (qualitative interview and audit data) provided further confidence in our findings.
Transparency of researcher assumptions is important to rigour:27 Two advanced practice liver nurses (SM, SS) who received structured, qualitative research training and mentorship (through the Sydney Community of Health Leaders And Researchers [SCHoLAR] program) perceived DVA as a common issue that rendered traditional venepuncture time consuming and painful. They believed EJV provided a safe, acceptable alternative but had no formalized data to confirm their assumptions.
The theoretical framing
The acceptability framework of Sekhon et al. was developed to understand and measure acceptability as a multicomponent construct. It explains how the components of acceptability of a health care intervention may intersect with other constructs such as engagement and adherence.24 Applying the 7 acceptability constructs to our intervention: affective attitude is how patients feel about having blood collection using EJV versus peripheral venepuncture, burden is the effort required to undergo the procedure, ethicality is the ‘fit’ with patients’ value system, intervention coherence is patients’ understanding of the purpose and mechanism of the procedure, opportunity cost captures what is given up to engage in this procedure, perceived effectiveness is how likely the patient feels that the procedure will work, and self-efficacy is their belief in their own capacity to engage in the procedure.
The 7 elements of Sekhon et al. are considered across 3 temporal zones: prospective acceptability explores the person's positioning toward the intervention before they experience it, concurrent acceptability explores experience while having the procedure, and retrospective acceptability occurs as the person reflects back on their overall experience (Figure 1).24
Results
People with DVA and liver disease recalled venepuncture experiences at 2 key time points: (1) participants’ recalled experiences of DVA prior to the availability of EJV (capturing acceptability of peripheral venepuncture) and (2) participants’ experiences of DVA after the implementation of EJV (capturing acceptability of EJV).
Acceptability of peripheral venepuncture
EJV recipients reflected on their experience of venepuncture prior to EJV availability (Figure 2). Six of 10 participants described onerous physical preparation for venepuncture, including physical preparation (hydration, setting aside time, exercise) and mental preparation (delaying, managing stress, anxiety, trepidation). Commonly described strategies included heat packs, ‘pumping’ muscles, and careful selection of 1 or a series of venepuncturists. These elements embodied the poor prospective acceptability of peripheral venepuncture, with preparation being a time-consuming burden that created an affective attitude of stress and avoidance. Bill explained:
‘I'd tell myself to relax, drink lots of water … then I'd get a stress ball and squeeze it to make the muscles work and help the vein. You think a lot about how to make it better … It was always stressful … it was like a mission!’
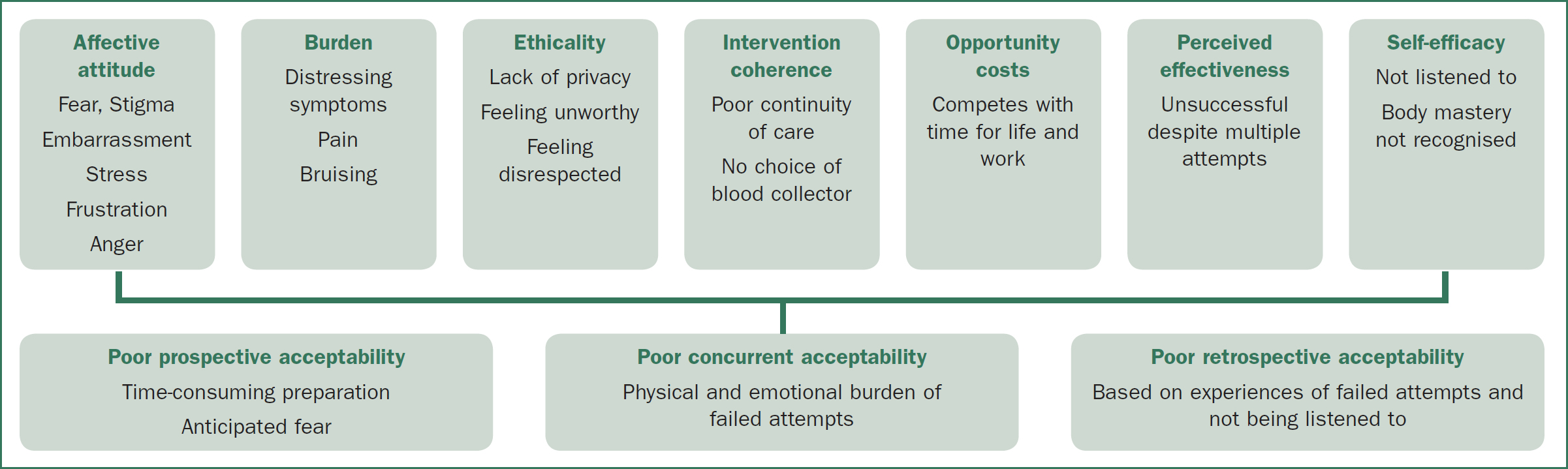
The prospective acceptability of peripheral venepuncture was influenced by poor perceived effectiveness and the participants' poor sense of self-efficacy, where despite their best efforts with physical preparation strategies, they were unable to contribute to success.
The physical burden of failed attempts resulted in poor concurrent acceptability of peripheral venepuncture. Even US guidance was unreliable. John explained:
‘Before it was an hour or 2 session, 2 or 3 people having a go … each one, 2 attempts … I could walk out with 8 holes in my arm and no blood. The next day, 3 or 4 people having another go … The doctor had a couple of goes … with [US] and failed … Sometimes I've had 3 docs try because they keep missing.’
Participants described negative affective attitudes, feeling stigmatized and embarrassed about multiple unsuccessful attempts. DVA led people to disclose their IDU history and hepatitis C status. They were then made to feel they were ‘just a junkie.’ Steve explained his affective attitude of stigma:
‘When the nurses take my … blood pressure and they see the track marks on my arm … instantly, their attitude changes … It's self-inflicted … I've [brought] it upon myself … It's upsetting in life that you get treated … not as a normal patient … Not equal.’
David also described poor concurrent acceptability of peripheral venepuncture through an affective attitude of stigma. During a 2-hour ordeal, he was conscious of his difference to other patients and questioned the ethicality and lack of privacy afforded to him and others receiving venepuncture in a shared space:
‘I was in this lab for 2 hours. They sit me in the corner with … hot towels and buckets of water … It was really uncomfortable. Patients are looking at me like, ‘Who is the guy in the corner?’ And they [staff] are, ‘Date of birth?’ to other patients. Really inappropriate! And between patients, they would try me again. It was really horrible!’
Anne, a liver transplant recipient, was troubled by the ethicality of admitting pain from peripheral venepuncture, feeling she needed to be worthy of her donated organ: ‘I felt I was so lucky to get the liver, I couldn't say anything to doctors … they would stick it in, and they'd be pushing around and hurting. I'd be nearly crying.’
Apart from anticipated fear around peripheral venepuncture, physical symptoms such as vasovagal dizziness was reported by both Mac and Dave: ‘Despite being a junkie for years … I'm now one of those people who feels faint when there's too much needle activity.’
Unsurprisingly, the shared meanings around the burden of peripheral venepuncture were that it was lengthy, painful, and frustratingly unsuccessful. The multiple attempts could encompass hours to days and multiple services. Outcomes included an impact on work-life commitments, bruising, and discomfort. David explained: ‘It generally turns into a traumatic experience with bruising and dead ends, clogged needles, and start again … it's painful and unpleasant.’
Although participants explained their DVA to blood collectors and made suggestions about what might work for them, their recommendations were dismissed or ignored, or clinicians took offense. This led to a range of negative affective attitudes: people felt frustrated, powerless, disrespected, and not listened to as they believed they knew their own body best. Guy explained:
‘ … if they'd listen! I said, ‘Just use a smaller needle’ … And they finally take it on board after they've stabbed you 3 or 4 times … It frustrates me … I've been injecting myself for 20 years … I've found every damn vein in my arms. So I know what's working and what's not.’
Patients described attending a known blood collection service and requesting a specific collector or planning their attendance for the time their preferred collector was available. This active preference for 1 person, favored for their past success and willingness to listen, caused tension between the patient and other clinicians. Despite their preferences, participants frequently described no choice about who collected their blood. There was a lack of continuity of care and knowledge to facilitate successful venepuncture. Guy revealed:
‘They'd say, “Oh [the regular blood collector] … she'll be back tomorrow.” I said, “I'm coming back tomorrow then.” She said, “We're out of appointments tomorrow.” … So they'd start, and after 3 or 4 stabs in this arm, they'd move to that arm. She says, “Have you ever had problems before?” … I said, “Yes … I've explained all this to the other lass. She knows my whole history.”’
As DVA was rarely handed over between visits, each time, there was a sense of starting all over again. The patient was not seen as a reliable source of information by clinicians, reducing their sense of self-efficacy. Even where there was a system in place to improve continuity of care, it was not used by clinicians. John explained:
‘There's … a [DVA] passport … a document you carry … I've got one of them … No one pays any attention to it … I've pulled it out a couple of times, and it's, ‘Yeah, don't worry about that. We'll be right.’’
While David developed a novel way of communicating an access point for peripheral venepuncture, clinicians’ lack of partnership reduced his self-efficacy and increased his stigma, making him feel discredited. The intervention coherence of persisting with ineffective peripheral venepuncture made no sense to him:
‘My GP in Sydney found a vein … and I went and got a tiny tattoo there … then I could tell people where to find blood … it's a great spot … but I live overseas now and … they will not go outside the box … I beg people, ‘Just roll up my pants. You'll … get blood immediately,’ and they're like, “No, I can't do it.” … And then it became weird and passive-aggressive. “Can I see the manager?” became, “You're going to have to leave the premises now”.’
The retrospective acceptability of peripheral venepuncture was then shaped by participants’ past experience of not being listened to. Jan explained how, as a master of her own body, her inability to be heard by clinicians reduced her self-efficacy, resulting in an affective experience of frustration and ongoing dread: ‘They won't listen. They won't believe you … You know they're not going to get it … So you dread coming to hospital.’ Sometimes this frustration led to an affective attitude of anger, with delays or disengagement with health services. Matt explained: ‘If I've warned them, it pisses me off because … they're still … ‘I think I can do it.’ … There's been times I've said, ‘Don't worry about it,’ and just walked out.’
The resulting disengagement with health services was one of several opportunity costs associated with the poor perceived effectiveness of peripheral venepuncture. David explained: ‘They'd say, “Go across town … to this lab”, where I'd already been and had failed … I never continued with those tests.’ The person's identity as a reliable worker was another opportunity cost arising from the poor perceived effectiveness of peripheral venepuncture. Guy described the time involved for multiple visits: ‘[They'd say], “Come back in a couple of days. Give your arms a chance to calm down’ … I said, “I work. I can't just be … nicking off for half a day.”’
With an extensive history of DVA, 3 participants described their fear of being in an emergency and not receiving the treatment they would need. Guy explained: ‘The worse part is my fear is that if I'm in a car smash … they'll say, “This guy's got no veins. What are we going to do?” I want them to go to my neck [EJV]!’
Acceptability of EJV
This time point was accessed by asking current EJV recipients to reflect on their experience when first offered EJV reflecting prospective acceptability, their experience during EJV reflecting concurrent acceptability, and what EJV now meant to them in the context of their life with DVA, representing retrospective acceptability (see Figure 3).
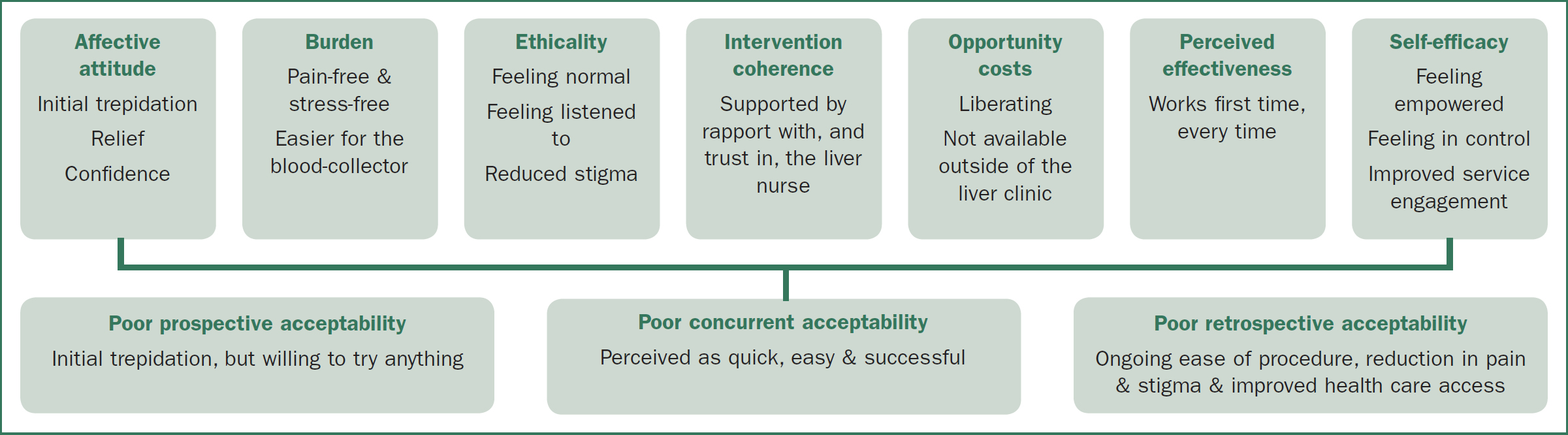
Several participants expressed their initial affective attitude toward EJV as trepidation. Their neck being used an access point was out of their scope of venepuncture experience, and they felt more vulnerable. John explained ‘At the very beginning, it was conceptually, “Oh, okay. That sounds kind of weird.”’ However, all participants found their initial concerns were groundless. Anne explained:
‘Going in your neck … at first, you're scared, and you think, “Oh, Jesus.” … But once they did one, that was it! … it was a bit scary, but … I thought, “What am I worrying about? It's fabulous. How cool is that?”’
Participants’ trepidation about EJV was overcome because, after the trauma of peripheral venepuncture, they were willing to try anything. Mac revealed: ‘If you'd had really bad experiences, you'd be open to [EJV].’ This poor acceptability of peripheral venepuncture strengthened the prospective acceptability of EJV.
Concurrent acceptability was defined by how easy participants perceived EJV during the procedure. This ease reduced the burden of venepuncture and was an enormous relief. David explained: ‘It was literally, “Hallelujah! Thank God for this!” It was one huge … part of the stress and anxiety out of the way.’ The ethicality or fit with participants’ value system was greatly enhanced. After the stigma of living with DVA, EJV gave people the opportunity to feel normal. John explained: ‘It's not quite like everyone else, but the end result is like everyone else.’ Participants described trust in the hepatology nurses; there was greater opportunity to build rapport with them through consistent care. The continuity of effective venepuncture through the liver clinic allowed participants to feel listened to and gave a sense of belonging. As Jan experienced: ‘Having … a group that understands you … They believe me and they listen.’ Anne concurred: ‘I'm very lucky … Everyone's so sweet and kind and laugh, and we all talk.’
The audit of 24 occasions of service in our DVA clinic revealed the median time taken for EJV was 5 minutes (range 1–15 minutes) compared to a median time of 15 minutes (range 5–30 minutes) for peripheral venepuncture, mitigating ‘time’ as an opportunity cost. There was a new sense of freedom arising from the certainty and timeliness of EJV. David explained:
‘[EJV] liberates you from … 3 things: stress, time, and pain. [Peripheral venepunture] is mentally stressful. It logistically takes up a bunch of … my time and the medical system's time, 40 minutes instead of 1 minute … and it's physically painful … Literally from the first time, EJV … is so completely painless.’
The perceived concurrent acceptability of EJV was driven by its perceived effectiveness. Participants went from multiple unsuccessful attempts through peripheral venepuncture to EJV working the first time every time with a rapid flow of blood. Mac explained: ‘Never had a problem … in the neck. Never! It's quick. Seriously, to have 10, 15, 20 vials of blood taken so quickly, it's a dream.’ This qualitative finding was supported by audit data where first attempt success rate for EJV was 100% (17/17) compared to 28.5% (2/7) for peripheral venepuncture (median 2, range 1–4 attempts). Participants also reported less bruising with EJV. Jan explained: ‘It was fine. I shut my eyes and just relaxed and just a tiny little prick … I was surprised I didn't even get a bruise.’
The retrospective acceptability was characterized by participants’ new sense of control over their situation. Guy expressed: ‘This makes my life easy, makes it easy for the nurse, and … she's not freaking out that she's stabbing and sticking and missing.’ This sense of control increased participants’ engagement with services. Jan explained: ‘For me, to know that I can go in … It's a good vein, can be got quickly, that's awesome! I'll come and do the bloods, where before, I'd, “Err … I'll come … in a week,” or [ask], “Who's on?”’
While EJV required a recumbent position, a benefit was the decreased resource utilization revealed by our audit data, which showed additional clinical assistance was requested more frequently with peripheral venepuncture (4 of 7 occasions of service), than with EJV (1 of 17 occasions of service).
A problem with retrospective acceptability was that EJV was not available in other venepuncture contexts. Patients found other services were usually unaware of the procedure, were unwilling to consider it, and seemed scared of the notion of blood collection from the neck. Participants wanted wider access to EJV. Jan lamented: ‘I just hope it becomes more available … that more people are up to doing it out of the neck and aren't scared by it.’ According to Bill: ‘I think there should be more nurses that could do it … They're changing patients’ lives.’ Jan suggested flagging patients who should have EJV preferentially: ‘Maybe we should get a bracelet or something … to state, “Do me neck” (laughs).’
Discussion
The acceptability framework of Sekhon et al.24 provided a rich theoretical structure to understand and report these important findings: while prospective, concurrent, and retrospective acceptability for peripheral venepuncture is poor, acceptability is high for EJV among people with liver disease and DVA. This heightened acceptability results from a reduced burden of pain, stress, and stigma, by feeling listened to and feeling liberated by a procedure that was quick and effective. Our findings address barriers identified in a recent systematic review including stigma and fear of venepuncture, disconnection, and distrust between health professionals and liver patients with DVA, and the impact of traumatic experiences of venepuncture on ongoing care.32
Participants’ descriptions of stigma were particularly moving. Goffman's seminal work33 defines stigma as a special kind of relationship between an attribute (the visible evidence of something) and stereotype (the collective social meanings we bring to that attribute). Two of Goffman's classifications of stigma that render a person as discreditable are ‘abominations of the body,’ mostly characterized by visible otherness, and ‘blemishes of character’ such as being ‘weak-willed.’ Many of our participants were viewed as having deformed veins, unable to render simple blood samples, and arms scarred with track marks from IDU, or all of the above. These are signs that convey social information to ‘normals,’33 and while usually concealed, the visibility of these stigmata is unveiled during traditional venepuncture.
Liver dysfunction is frequently associated with current or former lifestyle choices and with social deviancy or addiction. The venepuncture clinic provides the social space where deformity or deviance becomes visible so that the person with a stigmatized identity feels vulnerable to invasions of privacy.33
The consistent expressions of distress arising from inflexible blood collection practices resonates with Goffman's ‘structural stigma,’ embedded in institutional policies, practices, and attitudes.33 The high prevalence of hepatitis among culturally and linguistically diverse and indigenous patients leaves them open to Goffman's ‘tribal stigma,’33 compounding their sense of shame.
The significance of this heightened stigma is its association with psychological and social morbidity34 with lower health-related quality of life35 and as a substantial barrier to health-seeking behaviors.36 As DVA-related stigma seems largely avoidable given the demonstrated success of EJV, there is an imperative to increase the availability of this alternative approach. Our participants noted their access to EJV was limited to this single liver clinic, leaving scope for more comprehensive upskilling of venepuncture staff in other settings.
Anticipatory pain featured strongly in participant stories. The psychosomatic component of anticipatory pain arises from anticipatory cortical processes that heighten discrimination of painful stimuli and increase pain intensity.37 The insensitivity of clinicians to the pain and trauma of multiple venepuncture attempts in these patient stories was noteworthy. This apparent desensitization of some clinicians to nociceptive pain was confirmed in an Australian study, with multiple venepuncture attempts continuing despite obvious pain and distress.35 Complaints about rough, painful treatment or lack of accommodation of patients’ special needs continue to feature in national quality reports38 and may arise from poor communication between liver patients and clinicians.39
Acculturation among specialist clinicians is thought to contribute to shared beliefs about the intensity of procedural pain. Particularly where painful clinical tasks are routine, a tendency to play down patients’ pain may be a self-protective measure, particularly among clinicians with longer exposure.40 Given the mean reported venepuncture attempts in the general clinical environment is 1.25,41 the multiple attempts described by our DVA participants suggests a clear framework for admitting failure and seeking specialist input is lacking. As multiple venepuncture attempts are known to cause sympathetic activation, emotional distress, delayed treatment, and increased health care costs,4 EJV should be further investigated to confirm our findings of reduced nociceptive pain.
Conclusions
Peripheral venepuncture has poor prospective, concurrent, and retrospective acceptability and results in stigma, pain, distress, and burden for people with liver disease and DVA. EJV provides an alternative that is not only highly acceptable to patients but takes less time and uses fewer human resources.
Limitations
This study has limitations that may limit transferability. Firstly, it was conducted in a single center where advanced practice hepatology nurses had significant expertise in venepuncture and had close support from the Liver Transplant Anesthetist. Secondly, our audit data was conducted on a limited number of patients. While mixed methodologists are accepting of smaller datasets in the supporting arm,42 a larger quantitative dataset could strengthen the understanding of the impact of EJV on resource utilization.
Recommendations for practice
Three failed venepuncture attempts should trigger specialist referral, regardless of operator experience or qualifications, and the number of venepuncture attempts should be a routinely audited quality indicator.43 Where DVA presentations are common, services should consider revising protocols to support EJV implementation.

