A central venous access device (CVAD) is a type of intravenous catheter commonly used for administering fluids, parenteral nutrition, medications, antibiotics, and blood products. These types of catheters are common in both inpatient and outpatient care and may be used for short-to long-term therapies. Unlike peripheral intravenous (PIV) catheters, which extend only a short distance within the vasculature, CVADs terminate in the superior or inferior vena cava and can stay in place for longer durations. For many diagnoses, CVADs are considered lifelines.1,2
A known risk factor of CVADs are central line-associated bloodstream infections (CLABSIs), a major cause of morbidity and mortality.3 CLABSI is defined as a laboratory-confirmed bloodstream infection with a CVAD in place greater than 2 days and not related to an infection at another body site.4 It is estimated that one-third of the deaths caused by hospital-acquired infections are a result of CLABSI, and the National Healthcare Safety Network reported that an estimated 30,100 CLABSIs occur in United States acute care facilities each year.5
CLABSI impacts patients of all ages, but children appear to be disproportionately affected.6,7 In the neonatal and pediatric population, higher CLABSI rates occur in patients who are premature, critically ill, immunocompromised, or have intestinal failure.8,9,10 CLABSI rates in neonates have been reported from 3.2 to as high as 21.8 CLABSIs per 1000 central venous line days.7 Recent pressures brought on by the global COVID-19 pandemic have caused CLABSI rates to spike significantly, with the Centers for Disease Control and Prevention reporting a 65% increase in intensive care units (ICUs) and 47% increase across all location types in 2020.11
Even with infection prevention protocols and surveillance programs in place, CLABSIs remain a critical issue impacting patient outcomes and are often a focus for quality improvement efforts.12 The mortality associated with CLABSI ranges from 12% to 25%,11 despite the fact that most CLABSIs are considered preventable with the proper aseptic technique.13 The economic impact of CLABSI occurrences is significant, contributing to health care costs up to $46,000–55,000 per event to an estimated annual total exceeding $1 billion in the United States. Cost aside, there is a significant physical and emotional toll on patients and care providers who are faced with line removals, delayed treatments, increased exposure to antibiotics, and increased length of hospital stay up to 19 days.3,4,14
To decrease the risk of CLABSI, specialized care bundles are necessary.15 A care bundle is a set of evidence-based interventions that when implemented together have improved outcomes.16,17 Common components include hand hygiene, chlorhexidine skin antisepsis, use of maximal sterile barrier during CVAD insertion, and promptly removing catheters when no longer needed.18 Bundles are beneficial for standardizing CVAD care practices. To increase compliance, checklists have been embedded into electronic medical records for documentation of both insertion and maintenance bundles.4,17,19 Despite widespread adoption of bundled care practices, compliance can be a challenge, and CLABSIs remain prevalent in many care settings. In addition to monitoring compliance, practices and care bundles require ongoing review to ensure they are evolving with the latest evidence-based recommendations and advancements in medical technology.15,20
Causes of CLABSI
The pathogenesis of bacteria into the bloodstream can occur in two common ways: intraluminally and extraluminally. A CVAD can be colonized extraluminally by bacteria along the catheter or skin flora at the insertion site. Intraluminal contamination occurs at the catheter hub via add-on devices or introduced by an infusate.21 Haematogenous spread via the bloodstream from a distant site is less common.4 The pediatric population may have an increased risk for gross contamination events due to physiology and behaviors that are unique to younger patients.12 In babies and toddlers, common behaviors such as touching, pulling, and chewing on intravenous (IV) tubing and extension sets can increase the risk of a contamination event occurring. The proximity of soiled diapers and enteral feeding tubes to CVADs is often cited as a contamination risk by neonatal ICU (NICU) staff tasked with maintaining stable vascular access among the hospital's most vulnerable patients. Neonatal patients are particularly at risk due to immunosuppression, low birth weight, and underdevelopment.6,7
In older children, play and activity in the hospital setting is often encouraged but can result in IV tubing trailing behind on the floor or carpet, exposing vulnerable connections and hubs to pathogen entry.22,23 For pediatric outpatients, the many sources of contamination expand in the home and perhaps the school environment, as they carry out childhood daily living activities in the community, moving between caregivers with different levels of knowledge of caring for a CVAD.24
While adult patients are subject to many of the same risks for contamination, their ability to understand and adhere to infection prevention and hygiene best practices differs considerably. This notion that unique pediatric challenges may increase risk in children is supported by reports of a generally higher average CLABSI rate in pediatric than adult ICUs.6,7 However, CLABSI rates remain a critical issue in both adult and pediatric patients, suggesting a need for new and innovative solutions to be applied broadly across patient populations.
Many factors can influence a patient's risk for CLABSI: immune function, nutritional status, catheter location, and dwell time.25 For example, contamination risks increase with CVAD insertions in the subclavian, internal jugular, and femoral veins.4,6 The femoral vein is a common insertion site for critically ill children, and many of these patients have multilumen extension sets for delivery of their medications and solutions. The femoral vein is considered high risk for CLABSI due to contamination risks of stool and urine, skin folds, and high density of bioburden.26 It is difficult to pinpoint a cause or entry point for each case of CLABSI due to the multifactorial nature of the condition as well as differences in clinical practice, patient characteristics, and environments. However, a review of the literature suggests that efforts to strengthen CLABSI prevention bundles can make a difference, providing examples of interventions that decrease the risk of CLABSI.27
Focus on catheter hub contamination
Catheter hub contamination and microbial invasion of the skin insertion site are the most important and prevalent causes of CLABSI (Figure 1).28 Needleless connectors (NCs), now used on nearly all intravascular devices, were introduced with the intention of reducing needlestick injuries but came with the downside of being susceptible to microbial colonization, acting as an entry point for opportunistic pathogens to gain access to the bloodstream. In one study, 71% of CLABSIs were reported due to hub contamination.29 Another suggests that 50% of post insertion infections are caused by contamination of catheter hubs and NCs, establishing a strong link between catheter hub contamination and CLABSI.30,31 From the NC hub, microbes colonize the catheter lumen, form a biofilm, and increase the potential for CLABSI.30 It has been shown that a wide diversity of microorganisms are found on NCs. In fact, it has been reported that approximately 90% of NCs in use are colonized, mainly because of extraluminal contamination by skin flora and intraluminal contamination by manipulation.32

NC antisepsis, commonly referred to as the practice of scrubbing the hub, is essential for reducing contamination prior to accessing the CVAD. Chlorhexidine gluconate or 70% isopropyl alcohol are common antiseptic agents used for decontamination with scrubbing times varying from 5 to 60 seconds.30,33 Compliance to scrubbing the hub each time the device is accessed is critical, as most vascular access devices are accessed multiple times a day, with each instance potentially increasing the risk of microorganism entry and subsequent CLABSI.30,34,35 In recent years, use of disinfecting caps has grown as a method of protecting potential points of entry from colonization and transfer of pathogens to the bloodstream. Alcohol-impregnated port protectors provide continuous passive disinfection to NCs in addition to acting as a physical barrier to the NC between each line access.36
Even when accessed or protected with a disinfection cap, these connections or hubs are vulnerable to gross contamination from a patient's own body or the environment. Chamblee et al.12 defined contamination by bodily fluids as emesis, gastrointestinal secretions, oral or tracheal secretions, urine, or stool on the catheter threads, connectors, lines, or cap. For example, in patients with intestinal failure, gross contamination due to high stool output is a risk factor for CLABSI.9 Despite the passive disinfection and barrier protection by a cap, gross contamination events can result in visible contamination on catheter connection threads. This begs the question: Is scrub the hub and use of disinfection caps enough to protect catheter line-to-line connections and hubs from contamination?
The introduction of passive disinfection devices represented progress in the development of technology designed to protect the vulnerable points on CVADs from invasion by pathogens. However, clinicians continue to cite gross contamination of CVADs, connections, and NCs as a clinical problem and cause of CLABSI, suggesting that additional layers of protection may be needed to further minimize risk to the patient. While passive disinfection of catheter hubs with cap products has been studied and established as evidence-based practice,37 a protection gap remains, leaving the catheter system vulnerable to the many sources of gross contamination of bodily and/or environmental origin.
Clinical teams facing persistent CLABSI rates often improvise with off-label use of readily available medical or nonmedical grade products to address known or perceived gaps in protection. For example, off-label use of a flexible wax film wrap (Parafilm®) composed of a blend of waxes and polyolefins, intended for laboratory use sealing test tubes and beakers, to protect central venous catheter tubing connections in patients receiving parenteral nutrition or undergoing hematopoietic cell transplantation is documented in the literature.38,39 Together, these reports underscore the importance of protecting line connections and hubs from gross contamination and suggest that doing so can impact infection rates.
Other products that have been repurposed to be used as barrier protection for CVADs are medical tapes, kitchen cling wrap (Glad® Press'n Seal® Wrap), adhesive tapes, plastic bags, and clothing. While imperfect solutions, they serve the purpose of providing an added layer of barrier protection and emphasize the need for a safe, functional, and affordable solution. Ideally, barrier protectors used to guard lines should be single use and transparent as well as easy to apply and quick to remove to ensure safety is not compromised by adding the layer of barrier protection. Visible inspection of infusion lines is an important and routine part of clinical practice that allows for early detection of lines compromised by misconnection, medication leaks, breaks, contamination, or even missing or loose caps. From a usability perspective, simple and efficient use of the device is essential, especially now, as many institutions are facing strained staffing and resources.
A new solution
A novel Food and Drug Administration–listed device has become available recently to address this apparent gap in infection prevention technology and practice. Designed in collaboration with clinicians, the single-use transparent line guard (VALGuard® Vascular Access Line Guard, Covalon Technologies Ltd., Mississauga, Ontario, Canada; Figure 2) was developed in response to feedback from NICU personnel linking CLABSI cases in their practice to gross contamination events. Built with safety, efficiency, comfort, and cost in mind, the line guard has since been introduced at pediatric hospitals in the United States, implemented to enhance safety and standardize practice, moving away from makeshift solutions.
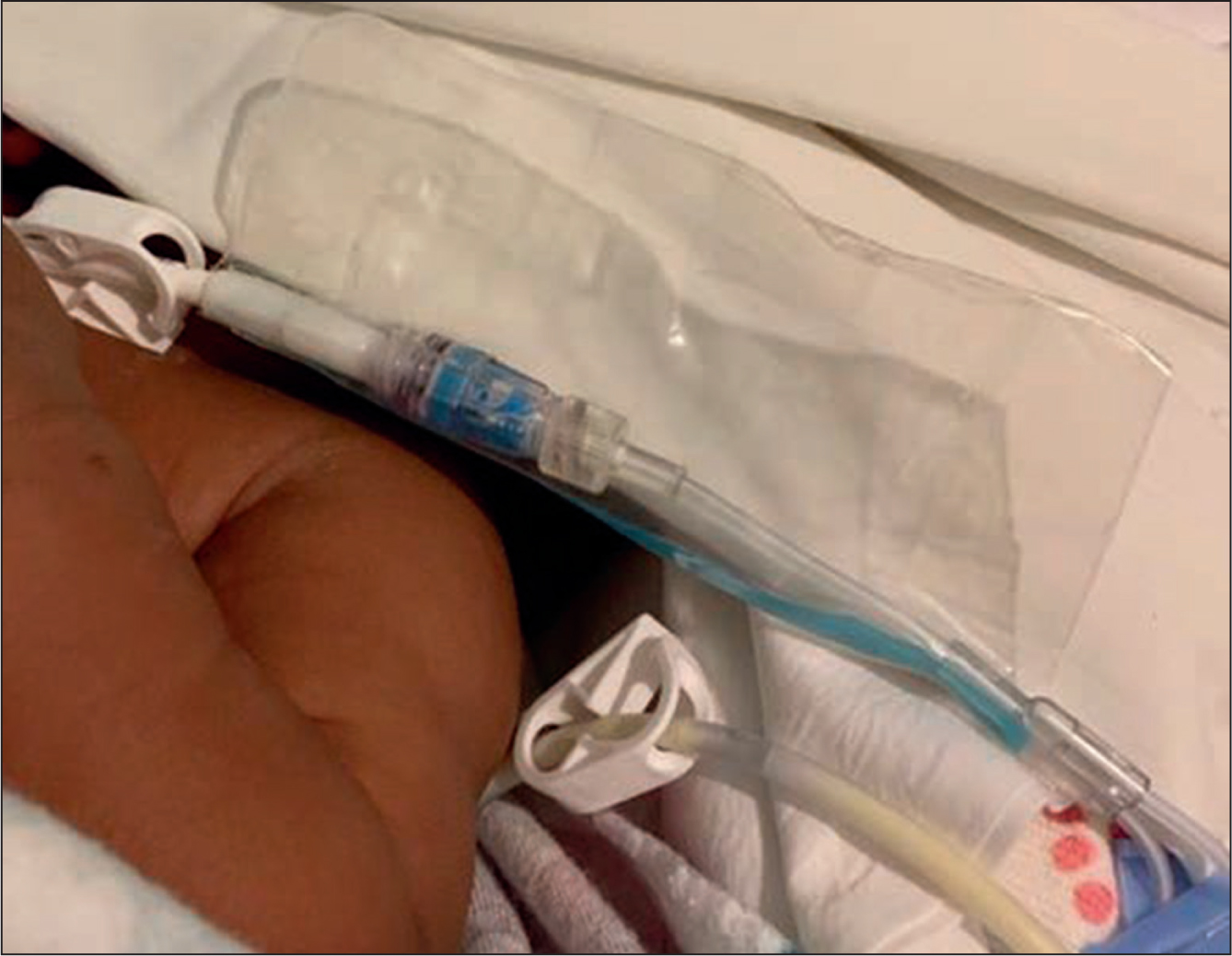
A quick-release pull tab incorporated in the design allows for quick removal, an important safety feature for maintaining fast access to infusion hubs in case of emergency, as well as for efficiency in normal practice which can require a CVAD to be accessed multiple times per day. The ability to quickly and easily apply and remove the barrier addresses a key challenge and safety concern with the various off-label solutions used to date. Wax film, tape, or plastic wraps, for example, can be especially tedious to remove from a line. It is a well-established rule that anything which may result in scissors being used around CVAD tubing should be avoided.
The product's transparency maintains visibility of CVAD connections and hubs at all times, allowing for clinicians, caregivers, or patients themselves to quickly detect a misconnection, leak, or contamination. Although the product was designed with the primary intention of keeping potential pathogens out, in some cases, keeping a barrier between the patient and the medication within the CVAD tubing is also a benefit. For oncology patients receiving chemotherapy drugs, for example, care must be taken to ensure patients and clinicians are not exposed to the drug outside of what is being delivered intravenously.
Functionally, the line guard is easy to use and incorporate into clinical workflow with basic training. It is available in two sizes that cover most connectors, including large manifold systems. Early adoption has been focused on the pediatric space due to a recognized need for a barrier and common use of the alternative solutions mentioned above; however, the product has widespread application in various high-risk patient populations for CLABSI. Compared with PIVs, CVADs are associated with an increased risk of infection which necessitates strict, multilayer infection prevention protocols and hygiene practices. However, increasing attention and research on the true contribution of PIVs to infection rates suggest that many infection prevention practices may be of benefit regardless of line type.40
Summary
The past few years have seen a sharp increase in CLABSI rates across settings, and more than ever, new solutions are needed to strengthen infection prevention bundles. Practical, cost-effective solutions that can be easily incorporated into current practice have the potential to improve outcomes without the burden of an added substantial cost or clinical time investment. Following innovation and introduction of a new solution, research is needed to determine the performance of the product beyond a case-by-case assessment. As such work is undertaken, we must lean on clinical expertise and an understanding of the pathogenesis of CLABSI to guide prevention practices. The actions of our peers and research on causes of CLABSI favor barrier protection where CVADs are vulnerable to pathogen entry, for which there is now a dedicated, safe, and cost-effective solution available.


