Intravenous (IV) access, both peripheral and central, is an integral part of the patient care pathways for diagnosing and treating cancer. Patients receiving systemic anticancer treatment (SACT) are at risk for developing infections, which may lead to hospitalisation, disruptions in treatment schedules and even death (Centers for Disease Control and Prevention, 2021). However, infection rates can be reduced and general patient outcomes improved with the evidence-based standardisation of IV practice, and the adoption of the appropriate equipment, such as peripheral IV cannulas, flushing solutions and sterile IV dressings (Easterlow et al, 2010).
Cancer treatment frequently involves the use of central venous catheters (CVCs)-also referred to as central venous access devices (CVADs)—which can represent a lifeline for patients when used to administer all kinds of IV medications, including chemotherapy, blood products and parenteral nutrition. They can also be used to obtain blood samples, which can improve the patient’s quality of life by reducing the need for peripheral stabs from regular venepunctures (Taxbro and Chopra, 2021). CVCs are relatively easy to insert and care for; however, they are associated with potential complications throughout their insertion and maintenance.
One serious complication of CVC use is catheter-related bloodstream infections (CRBSIs), which can increase morbidity, leading to prolonged hospitalisation and critical use of hospital resources (Akhtar and Lee, 2021). Early-onset CRBSIs are commonly caused by skin pathogens, and so a cornerstone of a CRBSI prevention is skin antisepsis at the time of CVC insertion. Appropriate antisepsis (decontamination/preparation) of the site for CVC insertion can prevent the transmission of such skin pathogens during insertion, while reducing the burden of bacteria on the CVC exit site (Loveday et al, 2014).
Evidence-based practice for the prevention of a CRBSIs and other healthcare-associated infections recommends skin antisepsis prior to insertion of a vascular-access device (VAD) using a 2% chlorhexidine gluconate and 70% isopropyl alcohol solution. This is recommended in guidelines such as epic3 (Loveday et al, 2014), the Standards for Infusion Therapy (Royal College of Nursing, 2016) and the Infusion Therapy Standards of Practice (Gorski et al, 2021). A strong evidenced-backed product such as BD ChloraPrep™ (Figure 1) has a combination of 2% chlorhexidine gluconate in 70% isopropyl alcohol that provides broad-spectrum rapid-action antisepsis, while the applicators facilitate a sterile, single-use application that eliminates direct hand-to-patient contact, helping to reduce cross-contamination and maintaining sterile conditions (BD, 2021). The BD ChloraPrep™ applicator’s circular head allows precise antisepsis of the required area, and the sponge head helps to apply gentle friction in back-and-forth motion to penetrate the skin layers (BD, 2021). BD ChloraPrep’s rapidacting, persistent and broad-spectrum characteristics and proven applicator system (Florman and Nichols, 2007) make it a vital part of the policy and protocol for insertion, care and maintenance of CVCs in specialist cancer centres such as the Royal Marsden. Meanwhile, the use of BD PosiFlush™ Prefilled Saline Syringe (Figure 2), a prefilled normal saline (0.9% sodium choride) syringe, is established practice for the flushing regime of VADs in many NHS Trusts.
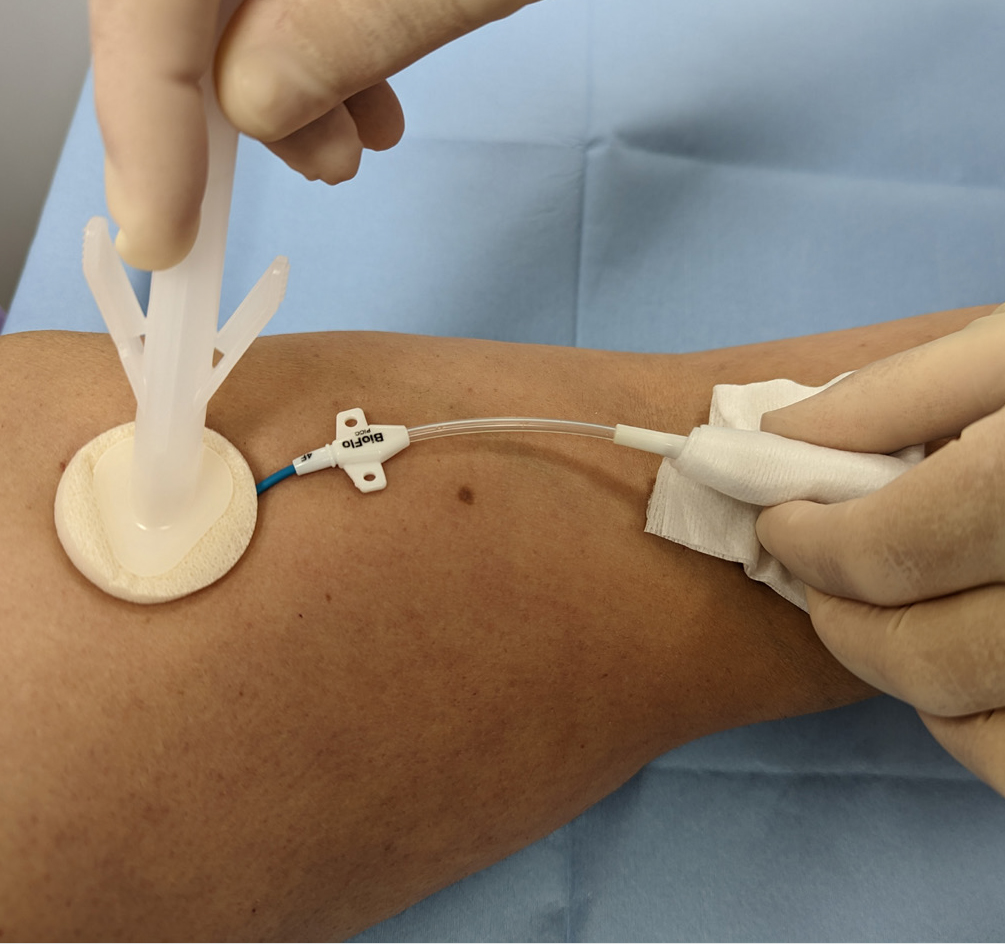
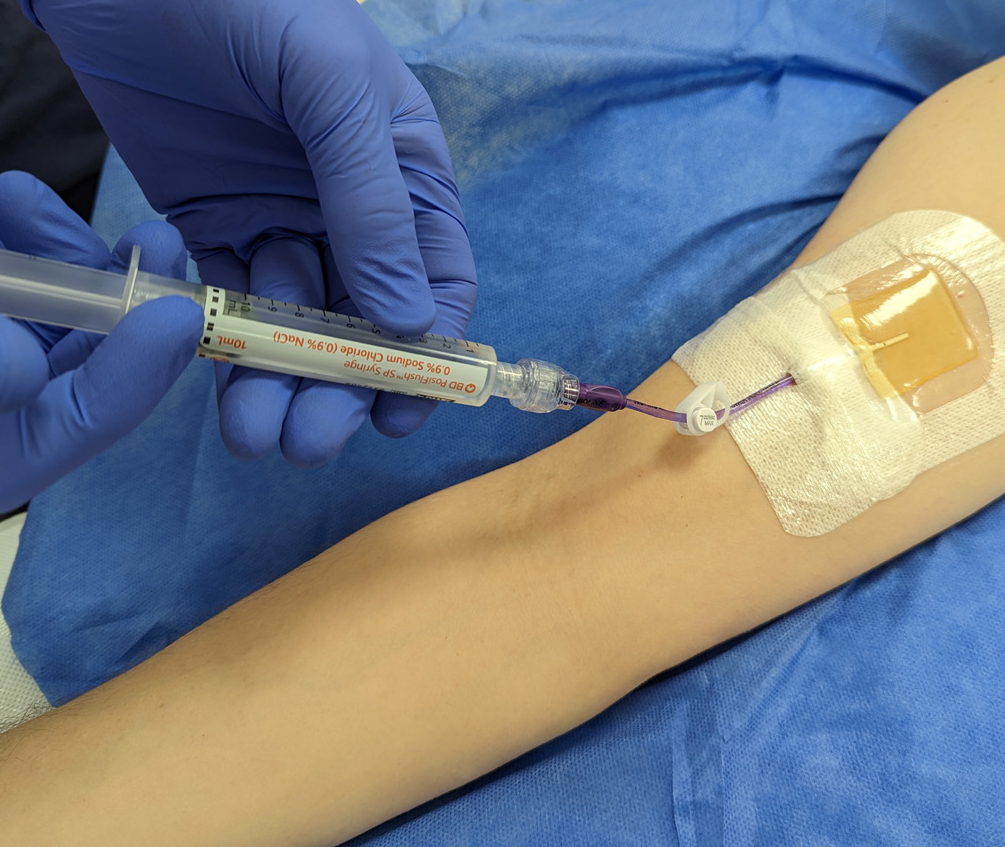
The following five case studies present examples from personal experience of clinical practice that illustrate how and why clinicians in oncology and other disciplines use BD ChloraPrep™ and BD PosiFlush™ Prefilled Saline Syringe in both adult and paediatric patients.
Case study 1 (Andy)
Gema Munoz-Mozas
Andy was a 65-year-old man being treated for metastatic colorectal cancer at the Royal Marsden NHS Foundation Trust specialist cancer service, which provides state-of-the-art treatment to over 60 000 patients each year.
Andy had a peripherally inserted central catheter (PICC) placed at the onset of his chemotherapy treatment to facilitate IV treatment. While in situ, PICCs require regular maintenance to minimise associated risks. This consists of a weekly dressing change to minimise infection and a weekly flush to maintain patency, if not in constant use. For ambulatory patients, weekly PICC maintenance can be carried out either in the hospital outpatient department or at home by a district nurse or family member trained to do so. Patients, relatives, carers and less-experienced nurses involved in PICC care (flushing and dressing) can watch a video on the Royal Marsden website as an aide memoir.
Initially, Andy decided to have his weekly PICC maintenance at the hospital’s nurse-led clinic for the maintenance of CVCs. At the clinic, Andy’s PICC dressing change and catheter flushing procedures were performed by a nursing associate (NA), who, having completed the relevant competences and undergone supervised practise, could carry out weekly catheter maintenance and access PICC for blood sampling.
In line with hospital policy, the PICC dressing change was performed under aseptic non-touch technique (ANTT) using a dressing pack and sterile gloves. After removal of the old dressing, the skin around the entry site and the PICC was cleaned with a 3 ml BD ChloraPrep™ applicator, using back-andforth strokes for 30 seconds and allowing the area to air dry completely before applying the new dressing. As clarified in a recent article on skin antisepsis (Clare and Rowley, 2020), BD ChloraPrep™ applicator facilitated a sterile, single-use application that eliminates direct hand-to-patient contact, which help reduce cross-contamination and maintaining ANTT. Its circular head allowed precise antisepsis around the catheter, and the sponge head helped to apply gentle friction in back-and-forth strokes to penetrate the skin layers.
Once the new dressing was applied, the NA continued to clean the catheter hub and change the needle-free connector. Finally, the catheter lumen was flushed with 10 ml of normal saline (0.9% sodium chloride) with a pre-filled saline syringe (BD PosiFlush™ Prefilled Saline Syringe). This involved flushing 1 ml at a time, following a push-pause technique, with positive pressure disconnection to ensure catheter patency. The classification of these syringes as medical devices enables NAs and other nonregistered members of the clinical team to support nursing staff with the care and maintenance of PICCs and other CVCs, within local policies and procedures. Using pre-filled syringes can save time and minimise the risk of contamination of the solution (Ceylan et al, 2021).
The use of pre-filled 0.9% sodium chloride syringes facilitates home maintenance of PICCs for patients. When Andy did not need to attend hospital, his PICC maintenance could be performed by a family member. Patients and relatives could access the necessary equipment and training from the day-case unit or outpatient department. Home PICC maintenance is extremely beneficial, not just to providers, but also to patients, who may avoid unnecessary hospital attendance and so benefit from more quality time at home and a reduced risk of hospital-acquired infections. Many patients and relatives have commented on the convenience of having their PICC maintenance at home and how easy they found using the ChloraPrep™ and BD PosiFlush™ Prefilled Saline Syringe ‘sticks’.
Case study 2 (Gail)
Simon Clare
Gail was as a 48-year-old woman being treated for bladder cancer with folinic acid, fluorouracil and oxaliplatin (FOLOX). She was admitted for a replacement PICC, primarily for continuous cytotoxic intravenous medication via infusion pump in the homecare setting. Her first PICC developed a reaction thought to be related to a sutureless securement device (SSD) anchoring the PICC. The device was removed, but this resulted in displacement of the PICC and incorrect positioning in the vessel (superior vena cava). Now unsafe, the PICC was removed, awaiting replacement, which resulted in a delayed start for the chemotherapy.
A second PICC placement was attempted by a nurse-led CVC placement team, and a line attempt was made in Gail’s left arm. Skin antisepsis was undertaken using a 2% chlorhexidine gluconate and 70% isopropyl alcohol solution (ChloraPrep™). A BD ChloraPrep™ 10 ml applicator was selected, using manufacturer’s recommendations, as per best practice guidance for CVC placement (Loveday et al, 2014) and to comply with local policy for the use of ANTT. The BD ChloraPrep™ applicator allowed improved non-touch technique and helped facilitate good key-part and key-site protection, in line with ANTT (Clare and Rowley, 2021).
The inserting clinician failed to successfully position the PICC in Gail’s left arm and moved to try on the right. On the second attempt, Gail noted the use of BD ChloraPrep™ and stated that she was allergic to the product, reporting a severe skin rash and local discomfort. The line placer informed the Gail that she had used BD ChloraPrep™ on the failed first attempt without issue, and she gave her consent to continue the procedure. No skin reaction was noted during or after insertion of the PICC.
BD ChloraPrep™ has a rapid-acting broad-spectrum antiseptic range and ability to keep fighting bacteria for at least 48 hours (BD, 2021). These were tangible benefits during maintenance of the CVC insertion site, in the protection of key sites following dressing change and until subsequent dressing changes. There are reported observations of clinicians not allowing the skin to fully dry and applying a new dressing onto wet skin after removing old dressings and disinfecting the exit site with BD ChloraPrep™. This has been reported to cause skin irritation, which can be mistaken for an allergic reaction and lead the patient to think that they have an allergy to chlorhexidine. In our centre’s general experience, very few true allergic reactions have ever been reported by the insertion team. Improved surveillance might better differentiate between later reported reactions, possibly associated with a delayed response to exposure to BD ChloraPrep™ at insertion, and local skin irritation caused by incorrect management at some later point during hospitalisation.
Staff training is an important consideration in the safe and correct use of BD ChloraPrep™ products and the correct use of adhesive dressings to avoid irritant contact dermatitis (ICD). It is worth noting that it can be difficult to differentiate between ICD and allergic contact dermatitis (ACD). Education and training should be multifaceted (such as with training videos and study days), allowing for different ways of learning, and monitored with audit. Local training in the benefits of using BD ChloraPrep™ correctly have been reinforced by adding simple instructions to ANTT procedure guidelines for CVC insertion and maintenance. Education on its own is often limited to a single episode of training, the benefit of using ANTT procedure guidelines is that they are embedded in a programme of audits and periodic competency reassessment. This makes sure that, as an integral part of good practice, skin antisepsis with BD ChloraPrep™ is consistently and accurately retrained and assessed.
Gail’s case illustrates the importance of correct application of BD ChloraPrep™ and how good documentation and surveillance are vital in monitoring skin health during the repeated use skindisinfection products. Care should be taken when recording ICD and ACD reactions, and staff should take steps to confirm true allergy versus temporary skin irritation.
Case study 3 (Beata)
Gema Munoz-Mozas
Beata was a 13-year-old teenage girl being treated for acute myeloid leukaemia. Although Beata had a dual-lumen skin-tunnelled catheter in situ, a peripheral intravenous cannula (PIVC) was required for the administration of contrast media for computed tomography (CT) scanning. However, Beata had needlephobia, and so the lead vascular access nurse was contacted to insert the cannula, following ultrasound guidance and the ANTT. After Beata and her mother gave their consent to the procedure, the nurse gathered and prepared all the equipment, including a cannulation pack, single-use tourniquet, skin-antisepsis product, appropriate cannula, PIVC dressing, 0.9% sodium chloride BD PosiFlush™ Prefilled Saline Syringe, sterile gel, sterile dressing to cover ultrasound probe and personal protective equipment.
Prior to PIVC insertion, a 4x5 cm area of skin underwent antisepsis with a 1.5 ml BD ChloraPrep™ Frepp applicator, with back-and-forth strokes for 30 seconds, and was allowed to air-dry. The vascular access team prefer to use BD ChloraPrep™ Frepp over single-use wipes, as the former is faster acting and provides the right volume to decontaminate the indicated area using ANTT (Clare and Rowley, 2021).
Following insertion, the PIVC was flushed with a 10 ml BD PosiFlush™ Prefilled Saline Syringe syringe, using a pushpause pulsatile technique, with positive pressure disconnection. Local policy recommends the use of pre-filled saline syringes, as they save time and minimise infection risk compared with manually drawn saline flushes (Ceylan et al, 2021). The Trust also permits competent non-registered members of staff to perform PIVC insertion, which is more cost-effective than depending on registered nurses.
In Beata’s case, the team considered the use of BD ChloraPrep™ and BD PosiFlush™ Prefilled Saline Syringe to be essential for the prevention of VAD-associated infections, as well as increasing the quality of nursing care by saving time in the day-case and inpatient settings alike.
Case study 4 (Emma)
Colin Fairhurst
Emma, a 43-year-old woman diagnosed with acute lymphoblastic leukaemia, was scheduled for an allogenic stem-cell transplant and associated chemotherapy. To facilitate this, she attended the vascular access service at University Hospitals Plymouth NHS Trust for the insertion of a triple-lumen skin-tunnelled catheter. This was identified as the best VAD for her needs, because of its longevity, multiple points of access and decreased infection risk compared with other devices, such as PICCs.
This was Emma’s second advanced VAD insertion, having previously received an apheresis line due to poor peripheral venous access, to facilitate the prior stem-cell harvest. She was yet to receive any treatment, and, therefore, no immunodeficiency had been identified prior to the insertion procedure.
Trust policy for skin disinfection prior to the insertion or removal of PICC lines is a 2% chlorhexidine gluconate and 70% isopropyl alcohol solution, BD ChloraPrep™. There is an exception for patient history of allergy or sensitivity to BD ChloraPrep™, where 10% povidone iodine is used instead. Emma had received BD ChloraPrep™ before, with no sign allergy or sensitivity, and so the vascular access team decided to use this product again for insertion. BD Chloraprep™ was used, in preference of other skin antisepsis options, due to the applicator’s ability to effectively penetrate the layers of the epidermis, as well as the ability to eliminate direct hand-to-skin contact between the operator and patient (Clare and Rowley, 2021).
Insertion of a skin-tunnelled catheter first requires disinfection of a large area, including the neck and upper chest. Following the manufacturer’s coverage recommendations, a 10.5 ml BD ChloraPrep™ applicator was selected as most suitable to cover an area of 25x30 cm (BD, 2022a).
The applicator was activated by pinching the wings to allow the antiseptic solution to properly load onto the sponge. To ensure proper release of the solution, the applicator was held on the skin against the anticipated site of insertion until the sponge pad became saturated. Then, a back-and-forth rubbing motion was undertaken for a minimum of 30 seconds, ensuring that the full area to be used was covered. The solution was then left to dry completely, prior to full-body draping, leaving the procedural area exposed for the procedure. Generally, drying time takes from 30 to 60 seconds, but local policy is not restrictive, as allowing the solution to fully dry is of paramount importance (Gunka et al, 2019). BD Chloraprep™ is effective against a wide variety of microorganisms and has a rapid onset of action (Florman and Nichols, 2007). Therefore, it was felt to be the best option for procedural and ongoing care skin asepsis in a patient anticipated to be immunocompromised during treatment.
It is the normal policy of the Trust’s vascular access service to flush VADs using BD PosiFlush™ Prefilled Saline Syringes with 0.9% sodium chloride. Likewise, BD PosiFlush™ Prefilled Saline Syringes Sterile Pathway (SP) are used to prime all VADs prior to insertion and to check for correct patency once inserted. BD PosiFlush™ Prefilled Saline Syringe were used in preference of other options, such as vials or bags, due to the absence of requirement for a prescription in the local organisation. They are treated as a medical device and, therefore, can be used without prescription. The advantage of this is that flushes can be administered in a nurse-led clinic, where prescribers are not always available. Aside from the logistical advantages, the use of pre-filled syringes reduces the risk of microbial contamination through preparation error and administration error through correct labelling (National Patient Safety Agency, 2007) In Emma’s case, three BD PosiFlush™ SP Prefilled Saline Syringes were used to check patency and/or ascertain venous location following the insertion of the skin-tunnelled catheter.
In this case, both BD ChloraPrep™ and BD PosiFlush™ Prefilled Saline Syringe proved simple to use and helped achieve a successful procedural outcome for the patient.
Case study 5 (Frank)
Colin Fairhurst
Frank was a 47-year-old man who had been diagnosed with infective endocarditis following a trans-oesophageal echo. A few days later, to facilitate his planned treatment of 6 weeks of intravenous antibiotics to be administered 4-hourly every day, he was referred to the vascular access service for insertion of longterm IV access. To facilitate this administration, the decision was made to place a PICC.
Frank’s referral included a history of illegal intravenous drug use and details of the consequent difficulty the ward-based team had in finding suitable veins to obtain vascular access. His medical history also included infected abscesses in the left groin and methicillin-resistant Staphylococcus aureus (MRSA) colonisation.
First, Frank was administered suppression therapy for MRSA decolonisation. Following this and prior to PICC insertion, the skin antisepsis procedure was undertaken using a 2% chlorhexidine gluconate and 70% isopropyl alcohol solution, BD ChloraPrep™, in adherence to Trust policy (Loveday et al, 2014). Specifically, BD ChloraPrep™ applicators are selected for their single-use application. They have been demonstrated to reduce the risk of infectious complications (catheter colonisation and local infection) by 92% compared with 5% povidone iodine (PVI) 69% ethanol (Guenezan et al, 2021). A 3 ml BD ChloraPrep™ applicator was considered suitable to decontaminate an area sufficient for the intended PICC insertion procedure, as recommended by the manufacturer (BD, 2022b). It was applied using a back-and-forth motion for a minimum of 30 seconds and left to fully dry (Loveday et al, 2014). Staphylococcus aureus bacteraemia’s have a mortality rate of 20-40% and are predominantly caused by VAD insertion (Ishikawa and Furukawa, 2021), and, therefore, the need to reduce this risk was of particular importance for this patient due to the history of MRSA colonisation.
In Frank’s case, the use of BD ChloraPrep™ during the insertion procedure and for each subsequent dressing change episode participated in an uneventful period of treatment. The clinical challenges posed by the patients’ presentation of MRSA colonisation meant the risk of infection was increased but, through correct antisepsis, no adverse events were noted, and the full course of treatment was successfully administered through the PICC.

