The suboptimal management of leg ulcers is a problem that involves increasing nursing resources and incurs high financial costs to the NHS (Guest et al, 2015; 2020). Guest et al (2015) identified a common pattern in relation to the failure to establish the underlying aetiology of leg ulcers, resulting in inappropriate treatment. To improve outcomes for leg ulcer patients, it is important to recognise the interconnected nature of the venous and lymphatic systems, identify lymphoedema in its early stages and address the lymphatics in parallel with the patient's venous condition (Wennen et al, 2019; Gasparis et al, 2020).
The assumption is that the predominant leg ulcer aetiology is venous. However, the data are more than 25 years old (Callam et al, 1985; Nelzén et al, 1994), with current evidence showing that most, if not all, patients with chronic venous insufficiency (CVI), stage 3 (CEAP classification (Zegarra and Tadi, 2023)) and greater, have lymphatic dysfunction (lymphovenous) or phlebolymphoedema (Prasad et al, 1990; Moffatt et al, 2004; Wennen et al, 2019). In western countries, lymphovenous oedema is the most common form of lymphoedema: it is a secondary lymphoedema that develops in patients with CVI; however, its impact is under-recognised due in part to the misdiagnosis of ‘chronic oedema’ (Moffatt et al, 2004; Bjork and Hettrick, 2019). The revision of Starling's law supports that all oedema is representative of lymphatic insufficiency (Levick and Michel, 2010; Mortimer and Rockson, 2014; Bjork and Hettrick, 2019). Chronic oedema associated with CVI is a symptom of both lymphatic and venous insufficiency (Farrow, 2010; Ellis, 2015; Moffatt et al, 2019). As such, optimal management of venous leg ulcers should embrace optimisation of not simply venous haemodynamics, but also lymphatic haemodynamics.
Most guidance or best practice statements relating to leg ulceration (Wounds UK, 2019; National Institute for Health and Care Excellence (NICE), 2023) does not mention lymphovenous disease in any detail and the relevance of lymphatic disease – its treatment is therefore not given the importance it merits. This can be seen within community care where it is estimated that 57% of patients who access community nursing services are estimated to have lymphoedema, many of whom are not able to access specialist services (Moffatt et al, 2004). Moffatt et al found that patients with leg ulceration have more complex aetiologies than was previously recognised. They observed uncomplicated venous ulceration in only 43% of cases; among the remaining patients, a large proportion had venous disease in combination with lymphoedema.
How does lymphoedema affect wound healing?
The main functions of the lymphatic system in the context of healing leg ulcers and the health of the integumentary system overall, which consists mainly of the skin, are the immune response and the management of extracellular fluid homeostasis, which occurs via the return of excess fluid back into the venous system from the lymphatic system (McGuire et al, 2022).
A continuous flow of lymph is needed to fight bacteria and prevent infection, as the white blood cells use the lymphatics to drag bacteria and toxins to the lymph nodes, which triggers an immune response. According to McGuire et al (2022):
‘Damage to or alteration of the free flow of lymph through local or regional lymphatic vessels in the area of a chronic wound contributes to pathological changes to the lymphatic system and dysfunction of chemical modulators resulting in a delayed immune response referred to as “lymphatic immunopathy”.’
The failure of the immune function of the skin is referred to as lymphostatic dermopathy (McGuire et al, 2022) and leads to the following:
- Loss of dermal integrity
- Tissue breakdown, with reduced oxygenation of the tissues and fibrosis
- Superficial infection and an increase in wound size
- Fibrosis that affects the nerves, which results in increased pain and discomfort
- The activation of inflammatory cytokines, which changes the oedematous fluid content from watery to protein rich (Wennen et al, 2019).
These lymphostatic changes, including lipodermatosclerosis, have previously been referenced as clinical findings associated with advanced CVI. The lymphostatic changes seen in CVI are caused by higher pressures in the veins, which increase filtration into the interstitial spaces. This eventually results in an overload of the lymphatic system, increasing oncotic pressure, leading to fluid being pulled back into the vessels.
Consequently, impaired lymphatics compromise the essential immune functions of the skin, making the skin itself and chronic wounds vulnerable to the following:
- High bioburden
- Chronic infections
- Recurrent cellulitis
- Recurrent ulceration.
When this inflammatory cycle becomes chronic, the ability to heal wounds is significantly decreased and the risk of developing wounds significantly increases (Wennen et al, 2019; McGuire et al, 2022).
The following case study highlights the complex nature of lymphovenous ulceration. It shows that changing the treatment pathway from venous management to an approach that targets the lymphatic system can lead to improved healing outcomes. This included modifying the application of compression bandages, treating fibrotic tissue, adapting antimicrobial pathways and wound bed preparation.
Case study
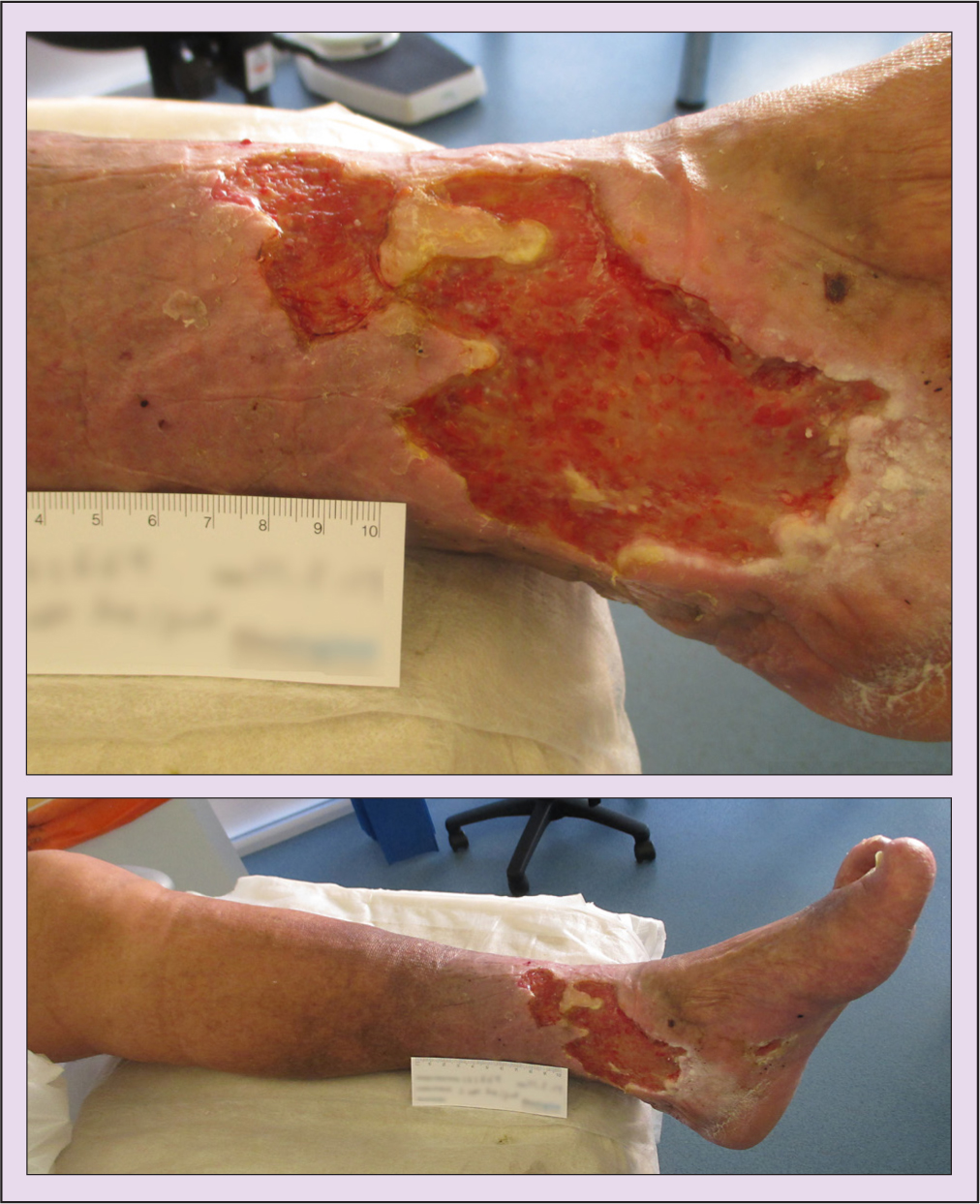
David Simpson (not his real name), aged 69 years, had leg ulceration to the left medial gaiter area. This was due to a trauma wound that he had sustained 20 years previously, which had recurred periodically. Mr Simpson presented at clinic with an ulcer he had had for more than 6 months, which had been treated by practice nurses. Figure 1 shows Mr Simpson's wound on presentation and its dimensions.
Medical history
Mr Simpson had previously had the following procedures:
- Vein stripping to the left leg
- Amputations to the second and third toe due to infection
- Metastatic prostate cancer with lymph node and bone involvement, for which he was commenced on chemotherapy midway through his treatment with Pioneer.
Physical assessment
The assessment identified that Mr Simpson had:
- Poor gait due to right hip pain and an imbalance to the left foot due to the amputations
- Late stage 2 lymphoedema, with positive Stemmer's sign to the toes (meaning that, on examination, it was not possible to pinch the skin between digits), with oedema to the foot, toes and above the knee
- Hard, fibrosed oedema with periwound fibrosis
- Severe hyperkeratosis
- A Pseudomonas wound infection (treatment with systemic antibiotics had already commenced)
- A Doppler ultrasound left ankle brachial pressure index of 1.23.
Treatment
The aim of treatment was to decongest the limb, soften the oedema and move the fluid to functioning lymphatic areas on the leg by applying modified compression and using lymphatic techniques such as mobilising foams, Kinesio tape and targeted compression. It also involved assisting the immune system to fight infection by following the steps of an antimicrobial pathway, as well as cleaning the wound bed with curettage to reduce the bioburden present due to the lack of lymphatic function and improve the condition of the skin to further reduce the risk of infection.
Clinical studies have shown that the effectiveness of compression therapy in CVI and lymphatic disease depends mainly on two factors:
- The interface pressure of the fabric on the affected leg (Mortimer and Browse, 2003; Torgbenu et al, 2023)
- The elastic property (stiffness) of the material, which determines the performance of the product when the patient is standing and walking (Partsch, 2005; Fletcher et al, 2013; Bjork and Hettrick, 2019; Ehmann, 2022).
It is clear from the research that a compression system should be individually tailored to each patient according to their ankle circumference and activity levels, as well as the size, duration and complexity of the ulcer(s) (Partsch, 2005; Fletcher et al, 2013; Bjork and Hettrick, 2019; Ehmann, 2022). It is also important to take into account the presenting texture of the skin, and whether it is hard or soft (Partsch and Mortimer, 2015; Bjork and Ehmann, 2019).
Mr Simpson was consequently commenced on a short-stretch inelastic bandaging system, which uses a stiffer bandage that provides a higher working pressure and lower resting pressure. Ehmann (2022) stated: ‘The working pressure is directly related to the resistance to stretch. It is this pressure dynamic that produces the “micro-massage” effect to stimulate a positive impact on the lymphatic, venous, and arterial circulation.’ It is therefore considered that the use of a stiffer material, which provides higher intermittent pressures, is more beneficial for healing ulcers in patients who have both venous disease and lymphatic disease (European Wound Management Association, 2005; Schuren, 2011; Mauck et al, 2014; Harding, 2015; Latz et al, 2015).
An 8 cm in width short-stretch bandage was applied in two layers in a figure of eight from the base of the toes to the ankle; a 10 cm bandage was next applied from ankle to below the knee in a figure of eight, and then another 10 cm bandage was applied in a spiral from toe to knee. Due to the position of the ulcer at the retromalleolar fossa, the following technique was used: narrow straps of a cohesive inelastic compression bandage were layered in a fan distribution over the ulcer and oedema to deliver higher compression to the area (Hopkins et al, 2011; 2013) (Figure 2).
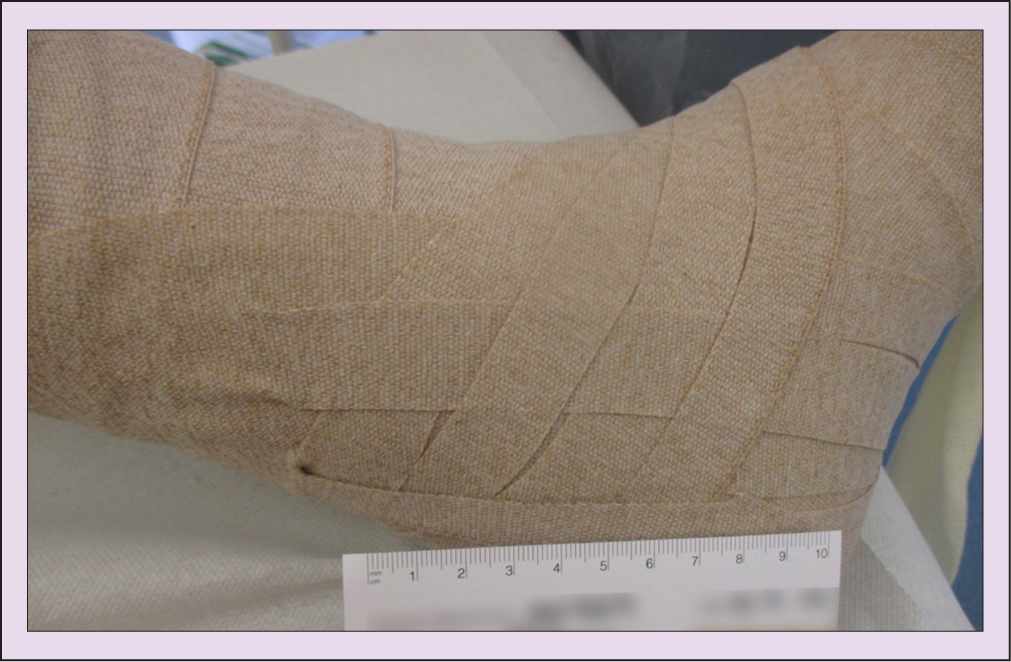
Toe bandages were applied to treat the toe oedema and Mr Simpson was shown how to undertake simple lymphatic drainage techniques to soften the oedema at the knee and move it to the lymph nodes at the groin.
The wound bed regularly underwent curettage to reduce the risk of infection, and an antimicrobial pathway was commenced. The fibrosis was softened through the use mobilising foams that massage the fibrotic tissue. These treatments need to be able to ‘decongest’ the limb by removing the extracellular fluid from the tissues to an area of functioning lymphatics – this movement through the lymphatic pathways helps reshape and soften the oedematous limb by removing the protein-rich fluid (Farrow, 2010; Ellis, 2015; Bjork and Hettrick, 2019). Kinesio tape correction strips were applied to the base of the toes to activate the flow of lymphatic fluid towards the lymph nodes; this technique works by lifting the skin to allow the lymph fluid to move and encourages contraction of the lymphangions to propel the fluid.
A skin care regimen was commenced consisting of an initial urea-based cream, followed by a liquid paraffin-based ointment and regular skin removal using a blunt-ended curette: the blunt end was used to scrape off skin cells and debris and the sharp end to debride the wound bed.
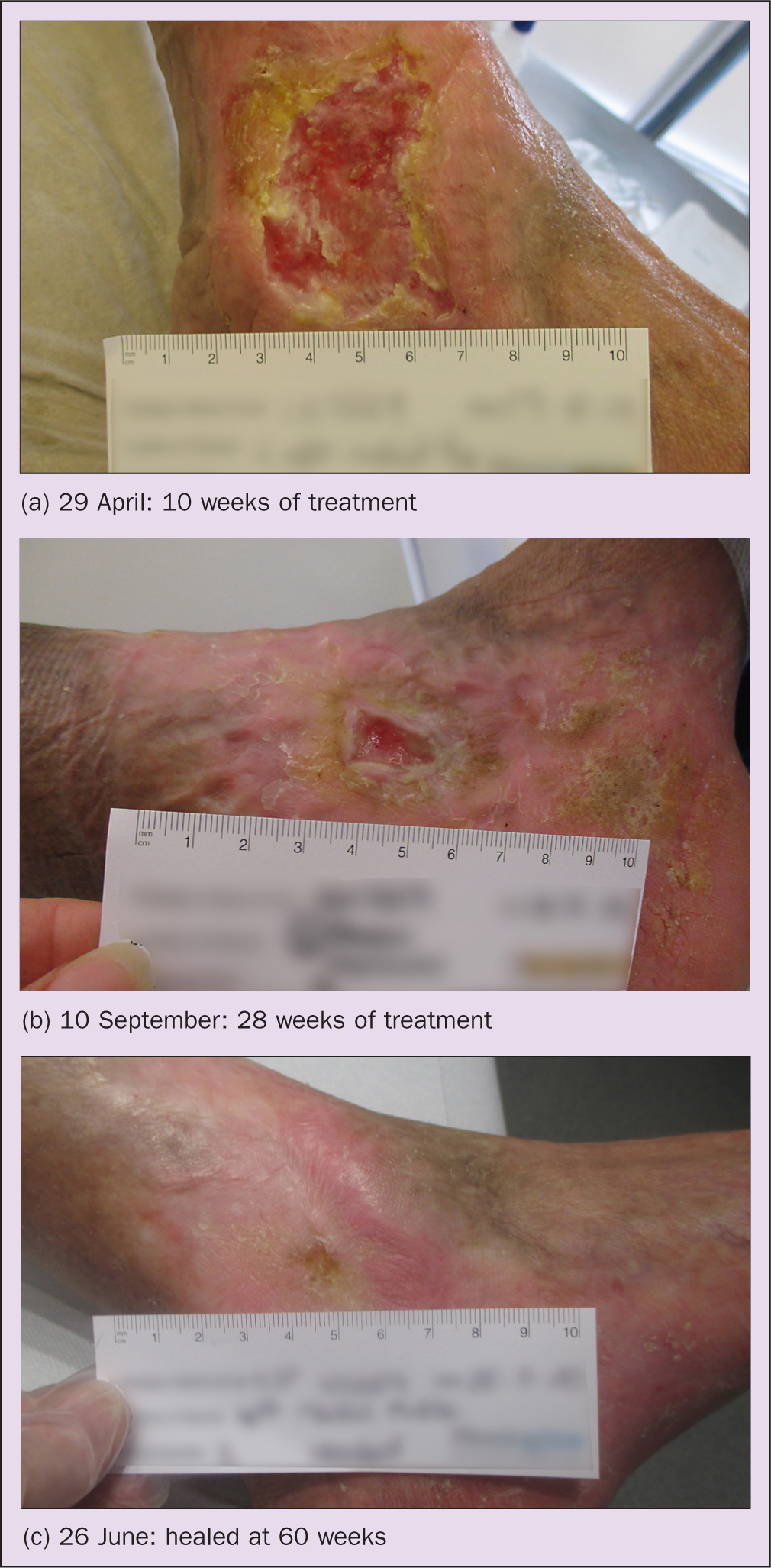
Figure 3 (a–c) shows the wound at different stages of treatment.
Discussion
David Simpson's progress was hindered by the commencement of chemotherapy. However, his leg ulcer eventually healed at 60 weeks (Figure 3c), with the periwound area significantly softened and the oedema reduced to the foot, toes and knee. This potentially would not have happened on a venous treatment pathway, which does not take into account the need to modify treatments to specifically target the lymphatic system.
The introduction and adaptation of complex decongestive therapy, consisting of compression therapy, simple lymph drainage and skin care, plus exercises to help mobilise accumulated oedema fluid and increase lymph flow, resulted in improved lymphatic drainage, as well as reducing fibrosclerosis (Földi et al, 2000; Elwell, 2015), as illustrated by this case study. Such an overall approach helps improve microcirculation – an essential component in wound care – and should be encouraged in order to improve outcomes in complex chronic leg ulceration caused by lymphovenous disease. Interventions to help prevent damage to lymphatic capillaries, and techniques to facilitate lymphatic drainage and lymphangiogenesis, need to be considered as part of routine wound management (Bjork and Hettrick, 2018). Such steps enable the complexities of lymphovenous disease to be addressed, especially in terms of the management of recurrent infections and fibrosis.
KEY POINTS
- Patients with lymphovenous disease have an increased risk of recurrent wound infection and are likely to experience reduced healing outcomes
- It is vital to undertake the correct assessment for the presence of lymphoedema, especially in patients with long-term chronic venous insufficiency
- The wound bed should be examined for signs of fibrosis, and a history of recurrent infection should be explored
- Patients with lymphovenous disease should be placed on a blended venous/lymphoedema pathway to ensure appropriate treatment
CPD reflective questions
- Can you recognise the presence of lymphoedema in your patients with chronic venous insufficiency?
- Do you understand the differences between compression types and application techniques, especially in relation to stiffness?
- Consider the importance of the lymphatic system in wound healing


