The skin is the body's first line of defence, protecting it against pathogens. Healthy skin is in a state of continuous renewal but extrinsic factors, such as epidermal stripping and tension blisters make the skin vulnerable (Flour, 2009). Patients in acute care settings are at risk of complications of infection, such as cellulitis, if the adhesive bond between tape and skin disrupts the epidermal barrier (Thomas, 2003). Furthermore, patients in critical care can be at a higher risk for infection and weak skin. Sepsis, a systemic inflammatory response from infection, is one of the leading causes of morbidity and mortality worldwide (Kleinpell et al, 2013).
Considering the importance of intact skin in preventing infection, nurses should attend to interventions surrounding invasive catheters, tubes and drains that will lower a critically ill patient's chance of acquiring a hospital-associated infection (Kleinpell et al, 2013). Promoting optimal patient care and interventions may involve exploring novel methods of meeting recommendations. Catheter securement with concurrent maintenance of safe skin can be a challenge in an intensive care unit (ICU).
This evaluation examined whether a nurse-constructed urinary catheter securement device using a silicone adhesive could reduce the complications of blistering and other skin breakdowns in a high-risk ICU population with Foley catheters. At the time of the evaluation, the nurse-constructed device was the only known catheter securement device that used silicone gel. Additionally, the commercially available devices used an acrylate adhesive, which can damage skin integrity (especially in patients with fragile skin), or non-adhesive straps placed around the thigh. For fluid-resuscitated patients, this presented a risk that the device could become a tourniquet. Research has shown that silicone tape is less damaging to the skin than paper tape, which has historically been considered hypoallergenic (Grove et al, 2013).
Literature review
The prevalence of indwelling urinary catheters in critical care units is considerably higher than the overall reported hospital-wide rates (Gray, 2010). Patients in the ICU frequently need catheters for hourly urine output measurements (Allen et al, 2020).
Patients in the ICU commonly have faecal incontinence, severe illness and impaired immune systems, which are some of the risk factors for catheter-associated urinary tract infections (CAUTI); one of the most common hospital-acquired infections (Gray, 2010; Amer et al, 2022). Even in general medical units, Foley catheters are so prevalent that it is estimated that up to 25% of inpatients will have one at some point during their hospital admission (Macneil et al, 2018).
In 2007, the Centers for Medicare and Medicaid Services designated CAUTI a reportable severe events – a non-reimbursable condition – from 1 October 2008 (Agency for Healthcare Research and Quality, 2015). Consistent with this directive, in 2009, the Healthcare Infection Control Practices Advisory Committee, a federal advisory committee to the Centers for Disease Control and Prevention, and the secretary of the Department of Health and Human Services published the Guideline for Prevention of Catheter-Associated Urinary Tract Infections (Gould et al, 2010). This document was based on a targeted, systematic review of the best available evidence and endorsed a category IB (supported by low-quality evidence or an accepted practice) recommendation that one should ‘properly secure indwelling catheters after insertion to prevent movement and urethral traction’ (Gould et al, 2010). The 2009 Healthcare Infection Control Practices Advisory Committee guideline also suggested that the evidence was poor regarding the efficacy of securing a catheter to prevent CAUTI (Gould et al, 2010).
The authors of an evidence-based report card conceded that ‘further research is needed to provide more robust evidence focusing on the potential of securement devices to reduce urethral trauma and CAUTI risk’ (Willson et al, 2009: 145). Despite the lack of evidence, the Society of Urologic Nurses and Associates (2010) included catheter securement to prevent urinary tract infections in its 2010 practice guideline. Additionally, in 2012, the Wound, Ostomy and Continence Nurses Society (WOCN, 2012; 2016) published a best practice guideline for urinary catheter securement that included a detailed list of the possible risks and benefits of using a catheter securement device, including irritation to the skin at the site of an adhesive securement device and reduction in trauma to the bladder neck and urethra.
Potential complications from an indwelling urinary catheter include infection, trauma, pain and skin issues related to securement. In one study, Foley catheter-associated trauma that required interventions ranging from prolonged catheterisation to cystoscopy was found to be as common as Foley catheter-associated symptomatic urinary tract infection (Leuck et al, 2012). The incidence of accidental catheter removal with a balloon inflated by the patient or staff in the ICU population has not been extensively studied. However, Lorente et al (2004) recommended implementing preventive measures to decrease the occurrence of this traumatic event after analysing the incidence of accidental catheter removal for all types of catheters in the ICU. Similarly, Shum et al (2017) found that there was a significant improvement in the incidence of catheter dislodgement and catheter-related pain on a general surgical unit when a device specific for urinary catheter securement was used versus non-sterile adhesive tape.
A Canadian case study identified a condition primarily affecting male patients with an indwelling urinary catheter. Dubbed Carignan's syndrome, it is characterised by postoperative burning and pain. Catheter traction was postulated to be the cause of this condition (Nelson and Carignan, 2000). This painful syndrome was reduced by anchoring the catheter with an adhesive securement device and hooking the drainage bag to the operating room table (Nelson and Carignan, 2000). Bell (2010) found that urethral erosion occurred when catheters were improperly anchored. She described four cases of older men in long-term care who developed partial to full-thickness erosions on the penis because their urinary catheters were either unsecured or secured improperly (Bell, 2010).
Like other medical devices, urinary catheters and securement devices have been associated with device-related pressure injuries. Since this type of injury can occur if any medical device is in contact with the skin and it is not unusual for urinary catheters to be used for extended periods of time in an ICU, prevention that includes regular moving or repositioning of the catheter and securement twice daily, especially if oedema is present, is in line with best practice (Gefen et al, 2022).
Skin irritation and epidermal stripping are documented as potential risks associated with adhesive catheter securement. (WOCN, 2012). In 2012, a consensus panel of experts from the US defined medical adhesive-related skin injury (MARSI) and offered prevention strategies (McNichol et al, 2013). The panel pointed out that silicone adhesive, unlike conventional adhesives, conforms more quickly to the skin and causes less pain and stripping of the epidermis on removal. However, the panel expressed concern that silicone might not be the correct adhesive to select when securing a critical device, such as a tube (McNichol et al, 2013). Additionally, they specified that ‘the more critical the device, the greater the need for the use of a higher-adhesion product and/or a stronger backing’ (McNichol et al, 2013).
In a study that analysed the performance of silicone tape by clinicians caring for patients with fragile skin, the results indicated that clinicians were highly dissatisfied with acrylic tapes for those with fragile skin (Manriquez et al, 2014). Konya et al (2010) looked at subjects aged ≥65 years living in a long-term care facility who required medical adhesive tape. They found that 15.5% of the subjects developed skin injuries that included contact dermatitis and blistering, notably near the margin of the tape (Shum et al, 2017). These results are similar to those seen in studies of blistering around the taped edge of postoperative dressings following total hip arthroplasty. A 13% incidence of blistering was found by Jester et al (2000) in the total hip replacement patients they studied; the lack of elasticity of the postoperative dressings and wound oedema contributed to blister formation. A significantly higher incidence of tape blisters (30%) was reported by Sellæg et al (2012) in a randomised clinical trial comparing different ways of draping in the operating room before hip replacement surgery. More tape blisters were observed among women than men (39% vs 19%; P=0.03)(Sellæg et al, 2012). The results of this study were inconclusive regarding the connection between the two methods of securing the strong adhesive on the surgical drapes to the patients studied and the resultant skin blisters (Bell, 2010).
This pilot evaluation aimed to determine whether a nurse-constructed urinary catheter securement device using a silicone adhesive could reduce the complications of blistering and epidermal stripping in a high-risk ICU population fitted with Foley catheters. This evaluation was a quality improvement project to MARSI, and all activities conducted by the authors were within the usual standard of care.
Methods
A prospective quality improvement evaluation was designed to investigate the incidence of MARSI associated with a standard acrylic adhesive urinary catheter securement device versus the incidence of MARSI using the nurse-constructed securement device. Since this was a quality improvement evaluation to reduce MARSI and all activities were conducted within the usual standard of care, formal institutional review board approval was not required.
In the large academic medical centre where this study was carried out, quality improvement initiatives are encouraged to improve patient outcomes. Hospital-acquired pressure injuries are a nursing quality indicator so the wound ostomy nursing team, managed by the first author (the principal investigator), routinely implements and evaluates quality improvements, which included this initiative. In addition, the nursing director of research confirmed this activity was exempt from needing ethical approval. However, all procedures complied with relevant laws and institutional guidelines.
A convenience sample of adult patients with Foley catheters in a surgical intensive care unit (SICU) was enrolled. This population was selected because of staff interest in preventing these frequently occurring wounds in the SICU. Criteria for inclusion included the presence of a Foley catheter and any degree of pitting oedema in the upper leg.
It should be noted that patients from the medical ICU are often placed in the SICU for bed capacity reasons. These patients were not excluded from the evaluation. Assessment for pitting oedema is standard for critical care nurses. It was done by applying pressure with a finger to the upper leg then observing if a pit or dimple was transient. The nurses' ability to perform this assessment was validated during orientation to the ICU unit. If a patient had a skin rash involving the thigh or groin, any skin breakdown on either thigh, or unilateral or bilateral above-the-knee amputation, they were excluded from the evaluation.
The principal investigator presented information about the evaluation to the SICU nurses during two staff meetings. The co-investigators also provided each nurse on the unit with one-to-one instructions before allowing them to enrol subjects in this evaluation. Each subject was assigned a unique identifying number that was recorded on the Catheter Anchor Trial (CAT) evaluation tool (Figure 1). The CAT tool also included: instructions for the nurse; a place to record the subject's medical record number; evaluation start date; pitting oedema scale; and oedema status if the securement device needed replacing (pink=nurse-constructed silicone adhesive; white=standard securement device) and why; and whether any skin breakdown was visible (Figure 1). Nurses were not required to place the devices on the same leg for all patients. This allowed for variability to meet specific nursing needs and patient needs.
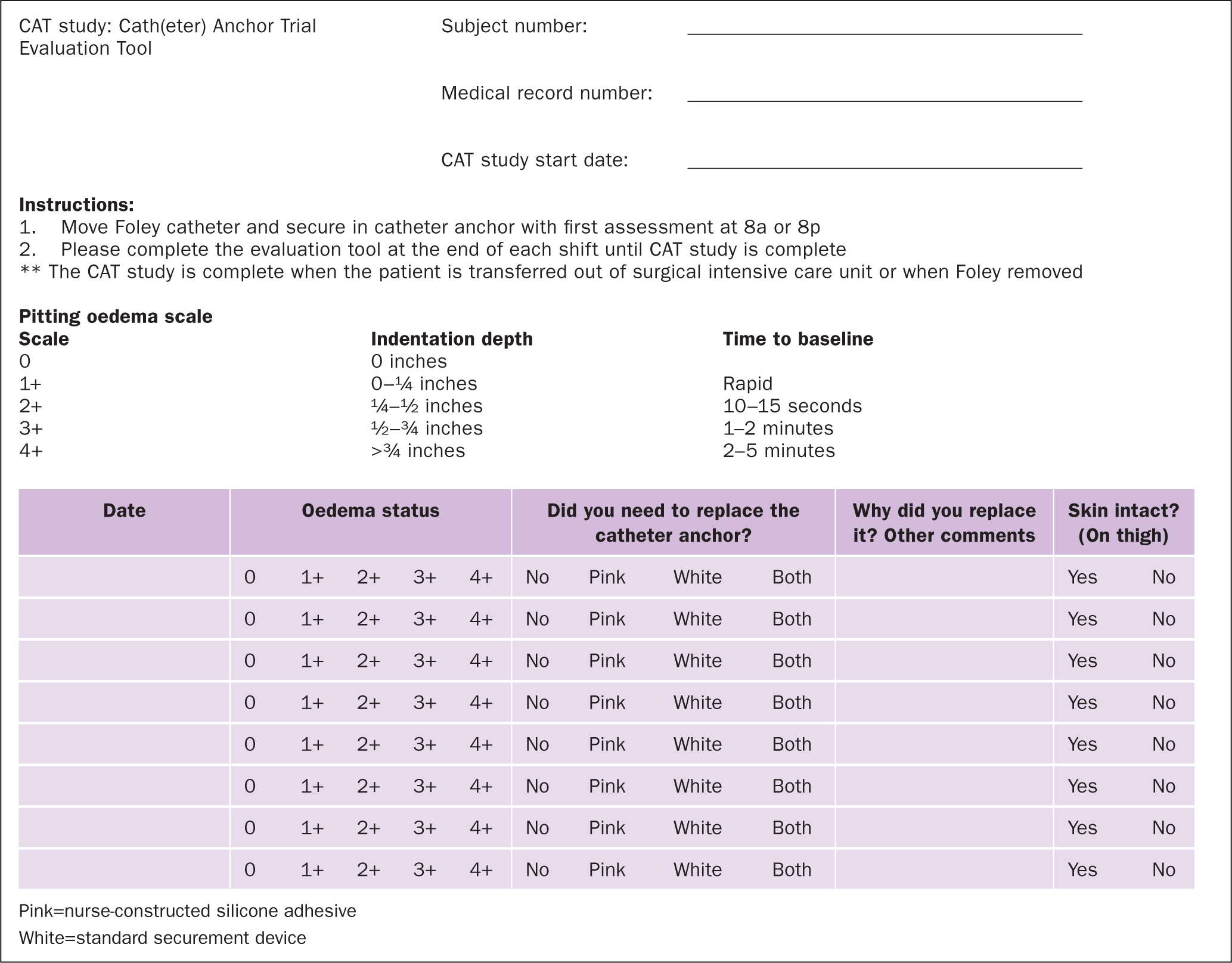
It was standard care in this institution for all inpatients with an indwelling urinary catheter to have an adhesive anchor device placed on the thigh to hold the catheter in place. Each subject had a standard securement device placed on one thigh (Figure 2) and a nurse-constructed silicone adhesive securement device placed on the contralateral thigh (Figure 3). At the beginning of each 12-hour shift (07:00–19:00), the nurses assigned to the evaluation would move the Foley catheter from one securement device to the other and record their findings on the evaluation tool. The principal investigator rounded in the SICU at least twice a week to be available to answer any questions the staff had regarding the evaluation and help identify eligible patients. In addition, the study coordinators, who were SICU nurses, regularly screened the patients to assess whether they met the evaluation criteria. Oedema was measured at the end of every 12-hour shift. As previously stated, the assessment was done by applying pressure with a finger to the upper leg then observing if a pit or dimple transiently occurred.
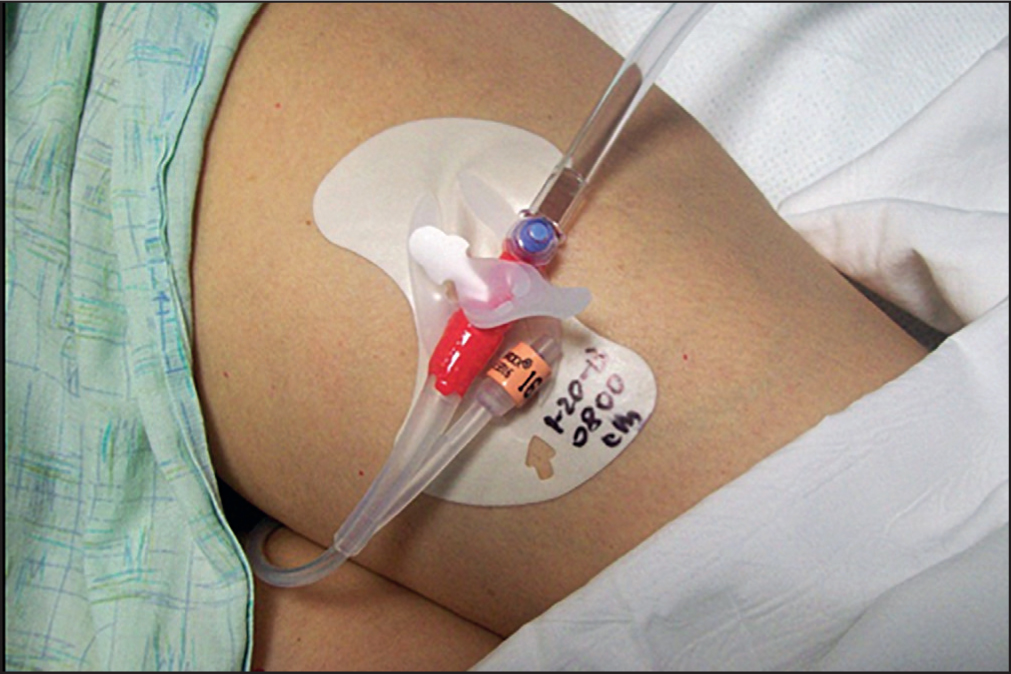
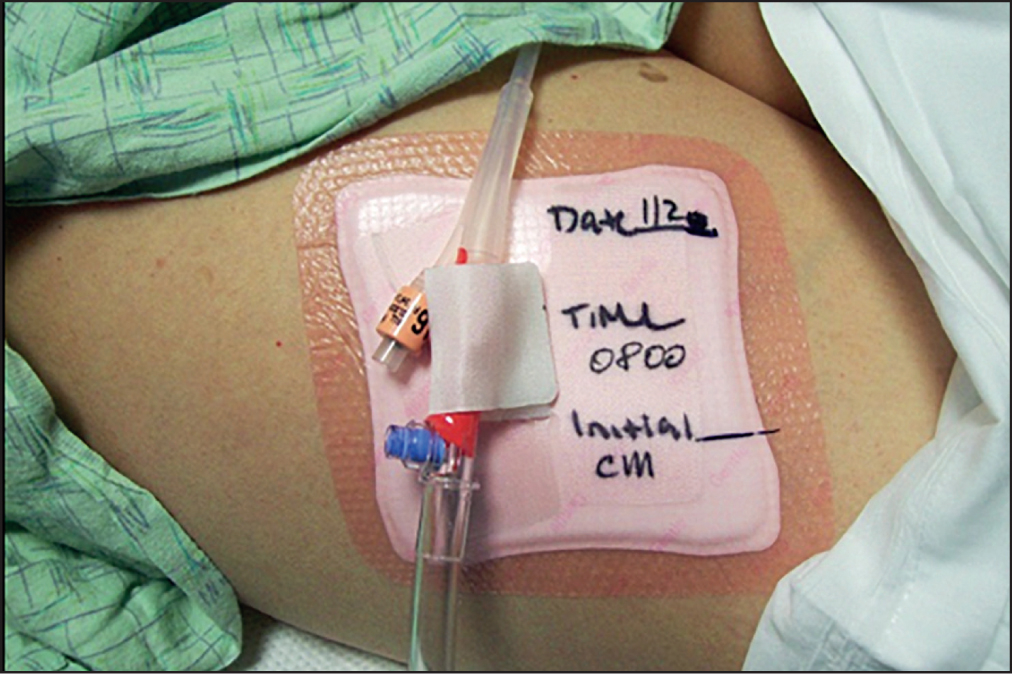
The standard Foley catheter securement device was an acrylic-adhesive-based product prepackaged with an alcohol wipe and skin sealant wipe to prepare the skin before the anchor was placed. The nurse-constructed device was a combination product that included an acrylic adhesive-based securement device taped to the outside of a silicone adhesive foam dressing; this alternative device fit better on top of the dressing and was also cheaper. All materials were available through the hospital's normal supply chain management department. The standard Foley securement costs three times as much as the alternate one secured to the foam dressing. The investigators used two separate products to create a new securement. Preparing the skin with alcohol or skin sealant under the nurse-constructed silicone adhesive securement device was not necessary.
Halfway through the evaluation, the investigators decided to change what securement device the Foley catheter was anchored with during the 07:00–19:00 hospital shift. Therefore, the last 14 patients had their catheters secured with the nurse-constructed device during the daytime and the standard securement device during the overnight shift. This was because some patients tended to be mobile during the day (especially since patients receive physical therapy during these hours) but not during the night. Investigators wanted to see how the securement device held up with increased movement, given the use of physical therapy during the daytime hours, so the change in the evaluation was made at the midpoint to ensure patients were using the nurse-constructed device during the daytime.
Given that this evaluation was a performance improvement project and all activities were within the usual standard of care, informed consent was unnecessary. However, family members were informed of what was being evaluated during their visits.
Analysis plan
A sample size of 35 was calculated using G*Power (Faul et al, 2007). All analyses were conducted using the SPSS v 25.0 statistical software package. Continuous measures (eg age) were reported as mean±standard deviation and categorical variables (eg sex) as a count (n; %) and c2. In addition, demographic and physiological characteristics of the groups (eg nurse-constructed catheter securement device vs standard catheter attachment) were compared using a Student's t-test to determine if there were differences between the groups. Statistical significance was set at P<0.05.
Results
There were 31 subjects enrolled in the evaluation with an average age of 61±6 (range 20–87) years and an equal percentage of men and women. Two patients were enrolled twice because they were discharged from, and readmitted to, the SICU. Their second admission data were discarded, yielding a final n=29. The mean length of evaluation stay was 12 days and did not vary across groups (sample: mean=12±14; skin breakdown: mean=16±10; no skin breakdown: mean=11±14; P=0.458).
In regards to the grading of oedema, there was visibly compromised skin integrity with an equal patient distribution across the four grades: 1 (21%); 2 (35%); 3 (24%); and 4 (21%). No skin breakdown was visibly associated with the nurse-constructed catheter securement device. Among those with a standard catheter attachment, n=23 (79%) had no skin breakdown, while n=6 (21%) had visibly compromised skin integrity (Figure 4). The mean age of subjects was not statistically significant between those who developed skin breakdown (65±10 years) and those who did not (60±18 years) from the standard adhesive catheter (P=0.540).
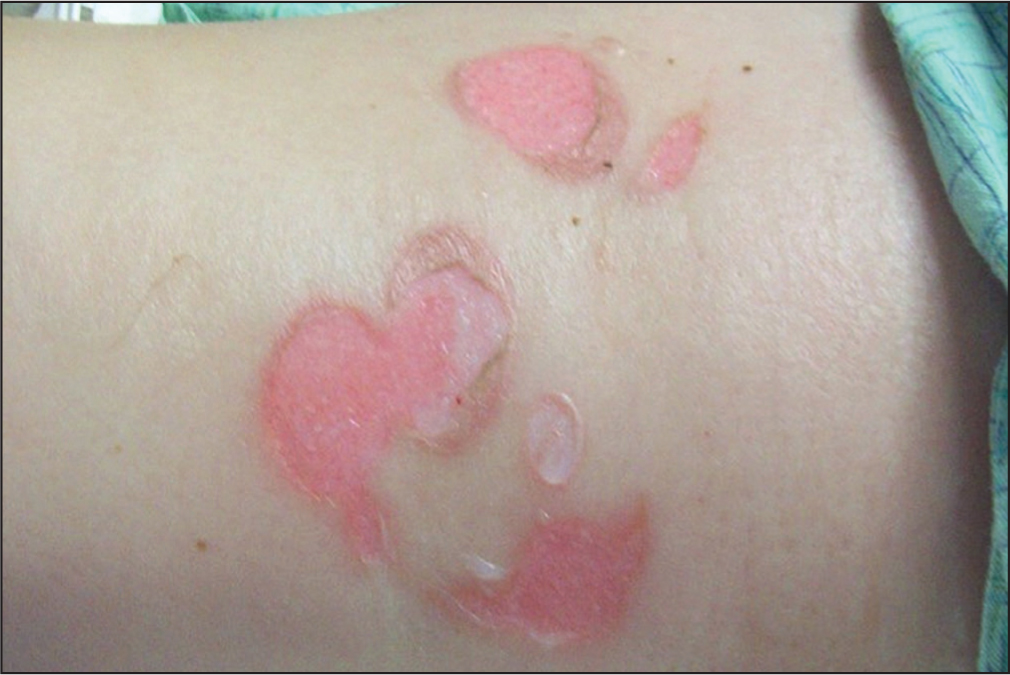
There was no statistical difference regarding the sex of those who developed skin breakdown from the standard catheter securement (P=0.928).
There was no statistical difference in the length of time measured in days for the patients who did and did not develop skin breakdown. The mean number of days with skin breakdown was 16±10, and the mean number of days without breakdown was 11±4 (P=0.458).
Using a χ2 analysis, a patient's oedema status was found to be a significant factor (skin breakdown=4; no breakdown=2; P=0.004). The mean oedema status at the beginning of the evaluation was 2±1 for patients who did not develop skin breakdown and 4±1 for those who had skin breakdown (Table 1).
Table 1. Skin breakdown
| Skin breakdown | |||
|---|---|---|---|
| Silicone gel adhesive | No | Yes | P |
| Number of patients | n=29 (100%) | n=0 | <0.001 |
Discussion
This evaluation was performed to compare the incidence of skin breakdown using a standard acrylic adhesive Foley catheter securement device versus a nurse-constructed device using silicone adhesive. The evaluation results demonstrated a significant incidence of skin breakdown using the standard commercially available acrylic adhesive-based securement and no breakdown visible with the nurse-constructed silicone-based securement. This finding is similar to that of Waring et al (2008) in a volunteer study; wound dressings with silicone adhesive removed less stratum corneum than acrylic adhesive-based dressings. It is also similar to what was found in a randomised clinical trial that compared two methods of draping hip replacement surgery and additional factors, including the type of dressing and number of dressing changes (Sellæg et al, 2012).
Sellæg et al (2012) found no significant difference in tape blisters after surgery in patients receiving the standard draping method versus a new procedure. The strong adhesive tape on the surgical drape and the acrylic-based postoperative dressing tape were discussed as relevant factors in the formation of tape blisters. This study also showed that using acrylic adhesive on a backing that does not stretch sufficiently when oedema develops causes tension blisters (Sellæg et al, 2012).
The cost of the nurse-constructed product was slightly higher than the standard securement without the silicone adhesive. However, the patients who developed the blisters or wounds from the standard securement experienced pain, which needed management plus dressing changes to treat the iatrogenic injury caused by the standard securement. This produced savings in the reduction of wound treatment costs.
In this evaluation, there were no differences regarding patient sex for the rate of skin breakdown associated with the acrylic adhesive securement. In the Sellæg et al (2012) study, there was a higher rate of tape blisters among women than men. There was also a significant difference in the amount of oedema in the patients who developed skin breakdown associated with their standard catheter securement versus those who did not exhibit a visible break in skin integrity. Patients with more oedema had a significantly greater incidence of recorded issues with skin breakdown.
Flour (2009) discusses vulnerable skin, including the stresses on the skin related to skin stretching, the ageing process and loss of elasticity and thinning of the skin, and the adverse effects on the skin from poor perfusion and oedema.
Although this evaluation was performed in the SICU, the results of this evaluation may be applied to any ICU patient with anasarca/leg oedema, not just those in the SICU.
Limitations
While this evaluation used a convenience sample of patients in the SICU and may be applied to any ICU patient, further evaluation in other ICU populations (eg medical, trauma and cardiovascular) should be investigated to ensure that results confirm what was found in this evaluation. This would increase the dataset's small sample size and strengthen the patient population's diversity to more than one ICU in a tertiary care academic medical centre.
Data were not collected on patient race. It is unknown from this evaluation whether ethnic background played a part in the development of skin damage from the adhesives.
The investigators used only materials readily available through the hospital's supply chain management department. Materials from other manufacturers may have different results.
Furthermore, the investigators may have been biased toward the nurse-constructed silicone-based securement and concluded data collection early based on early positive results. Further evaluation may be warranted to ensure that bias was not present.
Additionally, the nurse-constructed, silicone-based securement was compared to only one commercially available catheter securement device, which may reinforce investigator bias towards nurse-constructed silicone-based securement.
Similarly to Macneil et al (2018), this was an ex vivo evaluation.
Next steps
The investigators plan to expand this evaluation to create a formal randomised controlled trial to study the nurse-constructed silicone-based securement on different patient populations. Additionally, they plan to continue their development and refinement of a catheter securement device using a silicone adhesive.
Conclusion
A silicone adhesive urinary catheter securement device causes less skin damage than one using acrylic adhesive. One-step application, pain-free and atraumatic removal and reliable securement are essential considerations in development such products.
The evaluation findings suggest that further work in developing a Foley anchor using silicone adhesive may positively impact the incidence of skin breakdown associated with the adhesive material currently used for these devices.
Recommendations for future work include the design of a securement device that allows for a one-step application process, the ability to remove and reapply the device, and pain-free and atraumatic adherence and removal, plus the importance of reliable securement.
KEY POINTS
- Skin breakdown is common in intensive care units, and can be caused by adhesive on devices used secure urinary catheters
- A silicone adhesive urinary catheter securement device causes significantly less skin damage than one using acrylic adhesive but is not commercially available
- Women and men are at equal risk of skin breakdown associated with acrylic adhesive securement devices
- Oedema status is a significant factor related to skin breakdown
- One-step application, pain-free and atraumatic removal and reliable securement are essential considerations when developing device to hold a catheter in place
CPD reflective questions
- Why is it essential for patients in the acute care setting to maintain intact skin?
- What role does urinary catheter securement play in the prevention of catheter-related complications?
- How does silicone adhesive differ from the acrylic adhesive regarding to skin injury related to medical adhesives?


