Peripheral intravenous catheters (PIVCs) are the most commonly used invasive health device worldwide (Chen et al, 2021); as a result of this high demand, there is a multitude of products available, from various international manufacturers. Options for PIVCs include a retractable internal needle, to minimise needle-stick injuries (Prunet et al, 2008); blood control, which inhibits the reflux of blood into the open catheter hub immediately following insertion or on disconnection (Seiberlich et al, 2016); a stabilising platform, to minimise movement/rotation of the PIVC once in situ (Brimhall and Thorensen, 2004); and closed systems (integrated systems), which consist of a fused extension tubing to the primary PIVC, to minimise subsequent manipulation of attachments (González López et al, 2014).
Unfortunately, despite guidelines suggesting the preferential use of safety features, blood control, and integrated devices (Gorski et al, 2021) there is little evidence to guide healthcare services on their specific choice of device. PIVCs frequently fail before the completion of therapy due to complications including dislodgement (partial or complete), occlusion/blockage, infiltration (with or without extravasation), phlebitis, and infection (local or bloodstream)(Helm et al, 2015; Marsh et al, 2020). The sequelae of PIVC failure can include both negative clinical outcomes, such as compromised long-term vasculature, morbidity and mortality (in extreme cases) (Dychter et al, 2012; Saliba et al, 2018), and personal stressors for patients, such as pain, anxiety and dissatisfaction (Larsen et al, 2017).
With up to 69% of all PIVCs failing before completion of therapy (Bolton, 2010; Rickard et al, 2010; 2012), there is increasing demand for high-quality products to improve outcomes. One such example is the use of closed systems, also known as integrated systems. The first large randomised controlled trial (RCT) to compare the use of an integrated PIVC system with a non-integrated system (in a total of 1199 PIVCs), by González López et al (2014), found that patients with integrated systems were less likely to present with phlebitis (16.9% non-integrated v 12.0% integrated systems, P=0.01) and other complications (51.1% non-integrated v 42.6% integrated systems, P=0.004). Integrated systems, however, were less likely to be inserted successfully (98.1% non-integrated v 95.0% integrated systems, P=0.004), particularly on the first attempt (76.3% non-integrated v 66.0% integrated systems, P=0.001).
These findings suggest that, although establishing clinical efficacy in the reduction of PIVC complications, insertion failure and multiple insertion attempts may demonstrate potential complexity of device insertion or implementation difficulties with unfamiliar devices. To substantiate these claims, a similar large partially-blinded RCT (n=1759 PIVCs) was conducted in Australia (during 2018-2020) to compare integrated and non-integrated PIVC systems, which found all-cause failure (adjusted for other confounders) was significantly lower for integrated, compared with non-integrated PIVCs (hazard ratio 0.80,95% confidence interval per-protocol analysis 0.68-0.95) (Rickard et al, 2023). First-time insertion success was comparable between groups.
In the past, innovative PIVC designs such as injection safety and blood control features have resulted in positive practice changes and attitudes among nurses and other healthcare workers (Tarabay et al, 2016). However, implementation of new designs or devices sometimes involves learning curves and ongoing education to achieve the best possible patient outcomes in the long term. In the case of PIVCs, more complicated technologies such as ultrasound, although demonstrating efficacy (Stolz et al, 2015), have been difficult to implement due to the need for advanced education and practical training (Stolz et al, 2016). Methods such as virtual and simulated training techniques have become popular as a result (Adhikari et al, 2015; Torossian et al, 2019), however, staff attitudes continue to play a large role in effective implementation of innovative healthcare methods.
Overall, although integrated devices appear to demonstrate superiority in clinical outcomes, device differences may remain a potential barrier to implementation. The aim of this study was to assess acceptability to nurses of integrated PIVC systems, and to identify possible barriers and enablers for implementation of best practice.
Method
Study design
This study was undertaken as part of a large-scale, multicentre RCT investigating the efficacy and cost-utility of an integrated PIVC system in adult medical/surgical patients (Castillo et al, 2018; Rickard et al, 2023). Using a cross-sectional survey design, nurses’ experiences of, and level of satisfaction with, the integrated PIVC system were explored. The study is reported in accordance with the Consensus-Based Checklist for Reporting of Survey Studies (CROSS) guidelines for reporting survey studies (Sharma et al, 2021). Qualitative data in the form of free-text responses is reported using the Standards for Reporting Qualitative Research (SRQR) (O'Brien et al, 2014).
Setting and participants
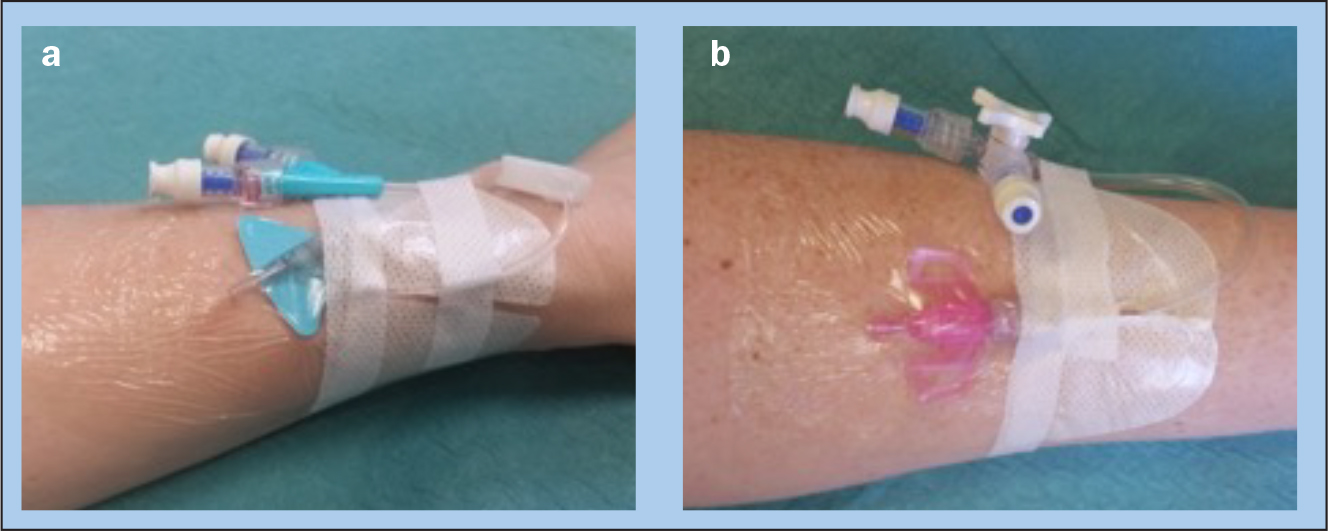
The survey was undertaken in Queensland, Australia, at two quaternary and tertiary referral teaching hospitals: the Royal Brisbane and Women's Hospital (929 bed) and Princess Alexandra Hospital (1050 bed), during June—September 2018. Participants were nurses caring for patients with an integrated PIVC (Nexiva Closed IV Catheter System Dual Port with SmartSite needle-free connectors; BD, Utah, USA) or a non-integrated PIVC (Introcan Safety 3 Catheter; B Braun, Melsungen, Germany) (Figure 1). A purposive sample of 100 nurses (50 per hospital) caring for patients with an integrated or non-integrated PIVC system were invited to participate in the survey.
Outcomes
The initial data collection form was designed by a clinical expert (NM) and underwent iterative review with the research team. The final survey included 17 items (including 6 free-text responses). The focus of the survey was on the key differences between the two systems regarding their function and appearance, and the subsequent differences in perceived comfort and injuries. Nurse-reported outcomes included perceived device securement (Did the wings prevent movement? Yes/No), perceived patient comfort when the system was placed in or adjacent to areas of flexion, such as the cubital fossa (Comfortable placing device in flexion area? Yes/No), observed injury or reaction related to device (Skin reaction or injuries? Yes/No), confidence dressing PIVC system (0=not easy to 10=very easy), ease of accessing the PIVC system (eg, delivering medications, flushing device; 0=not easy to 10=very easy), and overall confidence (0=not easy to 10=very easy).
Study procedures
The survey was disseminated in person by research nurses attending the wards. Nurses who were caring for patients with an integrated or a non-integrated PIVC device during a larger RCT (Rickard et al, 2023) were approached for participation once recruitment was well established, and asked to complete the survey based on the device they were currently caring for. Surveys were completed on a paper-based survey; surveys took less than 5 minutes to complete. Responses were entered onto a purpose-built Microsoft Excel spreadsheet by a research nurse.
Statistical analysis
Descriptive characteristics and survey results are presented using means and standard deviations or medians and interquartile ranges for continuous variables, and counts and percentages for categorical variables. Between-group differences were evaluated using t tests and chi-squared tests. An alpha value less than 0.05 was considered statistically significant. Data cleaning and analysis was undertaken using IBM SPSS Statistics for Macintosh, version 25.0. Free-text responses were analysed using a thematic synthesis approach, with responses organised into ‘descriptive’ themes in order to integrate key quotes with quantitative findings (Thomas and Harden, 2008).
Ethical considerations
Human research ethics committee (HREC) approval was granted by the Royal Brisbane and Women's Hospital HREC (HREC/16/QRBW/527), Griffith University Human Research Ethics Committee (Ref No. 2017/002) and the South Metropolitan Health Services HREC (Ref No. 2016-239). Consent was implied by completion of the survey.
Results
Participant characteristics
Between June and September 2018, 100 nurses completed the survey questions (100% response rate). Overall, most surveys were completed by surgical nurses (n=95) with more than 5 years of clinical experience (n=62) from the Royal Brisbane and Women's Hospital (n=87).
Nurses typically cared for more than 5 PIVCs per month (n=92) and were not credentialed to insert PIVCs (n=72) -ie, were not vascular access specialists. A summary of participant and device characteristics is outlined in Table 1.
Table 1. Participant and device characteristics
| Participant and device characteristics (n=100) | n (%) |
|---|---|
| Device | |
| Integrated | 50 (50) |
| Non-integrated | 50 (50) |
| Hospital | |
| Princess Alexandra Hospital | 13 (13) |
| Royal Brisbane and Women's Hospital | 87 (87) |
| Primary clinical field/area | |
| Surgical | 95 (95) |
| Coronary care | 5 (5) |
| Nursing experience (in years) | |
| <5 years | 38 (38) |
| »5 years | 62 (62) |
| PIVCs care per month* | |
| 0 PIVCs | 2 (2) |
| <5 PIVCs | 4 (4) |
| » 5 PIVCs | 92 (94) |
| Credentials to insert PIVC | |
| Yes | 28 (28) |
| No | 72 (72) |
PIVC=peripheral intravenous catheter
*Data missing for n=2 clinicians
Outcomes
Nurse-reported experiences of the devices are summarised in Table 2. Free text responses provided by nurses are included in Table 3.
Table 2. Nurse rating by device type
| Device | P value | ||
|---|---|---|---|
| Non-integrated PIVC system n (%) | Integrated PIVC system n (%) | ||
| Wings help prevent movement? | 0.019 | ||
| Yes | 27 (54.0) | 40 (80.0) | |
| No | 4 (8.0) | 1 (2.0) | |
| Unsure | 19 (38.0) | 9 (18.0) | |
| Comfortable using PIVC in flexion area? | 0.002 | ||
| Yes | 28 (56.0) | 39 (78.0) | |
| No | 13 (26.0) | 1 (2.0) | |
| Unsure | 9 (18.0) | 10 (20.0) | |
| Skin reaction or injuries? | 0.003 | ||
| Yes | 8 (16.0) | - | |
| No | 35 (70.0) | 47 (94.0) | |
| Unsure | 7 (14.0) | 3 (6.0) | |
| Confidence (applying dressing), median (IQR) | 7.0 (5.3-8.0) | 8 (6.0-9.0) | 0.113 |
| Tubing used, median (IQR) | - | 9.5 (8.0-10.0) | - |
| Ease of access, median (IQR) | 8.0 (6.3-9.0) | 10.0 (9.0-10.0) | <0.001 |
| Overall confidence, median (IQR) | 8.0 (7.0-10.0) | 10.0 (8.3-10.0) | <0.001 |
IQR=Interquartile range; PIVC=peripheral intravenous catheter
Table 3. Additional comments and feedback from nurses
| Device characteristic | Device | |
|---|---|---|
| Non-integrated PIVC system | Integrated PIVC system | |
| Wings for securement | - |
|
| Comfortable in flexion area |
|
|
| Skin reaction or injury |
|
|
| Confidence |
|
|
| Overall confidence |
|
|
| Further feedback |
|
|
dx=dressing; IVABs=intravenous antibiotics; IVC=intravenous catheter; PIVC=peripheral intravenous catheter; pt=patient
Device movement
Most nurses reported that the wings on the integrated PIVC system prevented device movement (n=40, 80%, P=0.019), with one nurse commenting that the device was ‘more secure’. In comparison, significantly fewer nurses reported that the wings of the non-integrated system helped to prevent movement (n=27, 54%). Additionally, a larger group of nurses (n=19, 38%) were unsure if the non-integrated system prevented movement overall.
Device comfort
Similarly, a significant majority of nurses reported patient comfort using the integrated system in an area of flexion (n=39, 78%, P=0.002), with one participant providing feedback that the device ‘gives extra movement for the [patient]’. Overall, 56% of nurses reported patient comfort using a non-integrated system in an area of flexion (n=28). Of those nurses who reported that this system was not comfortable, feedback included: ‘Hard and rigid plastic negative to [patient's] skin if in cubital fossa etc’.
Injuries
Most nurses reported no skin reactions or injuries for either the integrated (n=47, 94%) or non-integrated (n=35, 70%) systems; skin reactions and injuries were reported in the non-integrated group only (n=8, 16%, P=0.003). Feedback for the non-integrated system included concern about a higher risk of pressure injury (‘Pressure indentation under [device] wings when [PIVC] removed’; ‘Pressure areas — none that have passed Stage 2 however’), particularly in patients with frail skin. Other comments included noticing ‘marks’ and ‘redness’. In comparison, one nurse in the integrated group commented that ‘Our current ones do cause pressure areas from wings — these don't’.
Ease of use and confidence
Median ratings for ease of access were significantly higher for the integrated compared to the non-integrated (P<0.001) system, as was the median rating of overall confidence using the device (P<0.001). In terms of confidence, one nurse reported that for the non-integrated system, they ‘often use [extra secure dressing] for reinforcing or bandage over top’, while another reported the device was ‘Difficult to hold when inserting’. Nurses provided feedback that other factors impacted confidence for both systems, eg, ‘The [integrated device] is fine, but if the skin is wet/hairy this will obviously affect its integrity’, and ‘…depends on staff action (shaving around [non-integrated device] site)’. Nurses also provided feedback that ‘The design of this [integrated system] makes it less likely to be dislodged’, and, ‘This is particularly good from the perspective of inserting the PIVC’, for the integrated PIVC system.
Discussion
This study aimed to assess nurse-reported acceptability of the integrated PIVC systems. Primarily, the nurses providing feedback were not vascular access specialists but they were routinely required to provide care for patients with PIVCs in situ. Previous research has emphasised that understanding end-user knowledge, observations, and preferences when adopting new clinical practices is essential for ensuring both user acceptability and the successful implementation into routine practice (Franklin et al, 2012; Tarabay et al, 2016). In this study, clinicians’ overall level of satisfaction with the integrated PIVC system was significantly higher compared to the non-integrated PIVC system; consistent with other studies (Galang et al, 2020). Additionally, nurse-reported confidence in the dressing, tubing and clamp used with the integrated PIVC system, and overall ease of use and confidence, was ‘very easy’, indicating few barriers to the use of the integrated system. The high overall confidence and perceived ease of use should therefore facilitate the implementation of this system in clinical practice. In comparison, although nurses reported similar ratings of confidence in dressing the non-integrated PIVC device, ease of use and overall confidence was significantly lower.
Overall, none of the nurses using the integrated system reported skin injuries in their patients. In comparison, 16% of nurses who used the non-integrated system reported skin injuries in their patients, which was reflected in the feedback provided about risk of pressure injury concerns. Although pressure injuries and skin damage associated with intravenous therapy are well-documented (Thayer, 2012; Hitchcock and Savine, 2017; Ullman et al, 2019), adverse skin reactions potentially related to dressing and securement (eg, itching, rash, blister, skin tear, and bruising) when using integrated versus non-integrated devices are not well understood. Results from this study indicated that nurses perceived greater numbers of skin injuries in the non-integrated system, and further research to explore this finding is needed.
Additionally, much of the focus on device securement has centred on dressing securement rather than device characteristics; eg, in their study, Bausone-Gazda et al (2010) compared integrated and standard PIVC devices. However, they were focused on comparing securement-related complications between a standard PIVC without dressing for securement, and an integrated PIVC device with wings, plus a specially designed Tegaderm dressing for securement. In the current study, nurses who used the non-integrated PIVC system reported that the wings of the device did not prevent movement, or uncertainty at the wings preventing movement, at significantly higher rates compared to the integrated PIVC system. Nurses reported uncertainty related to the effectiveness of wings for securement, however, for both PIVC systems. The reason for this was unclear from feedback, and determining whether this is due to a perceived higher profile may warrant further evaluation. Overall, this finding suggests that perceptions regarding the device wings may still create a barrier to its implementation into clinical practice.
Most nurses reported patient comfort in an area of flexion for both devices, although this was significantly higher for the integrated system. Despite this, the study identified that a minority of nurses were unsure about the comfort of this system in an area of flexion. Typically, placement of PIVCs in areas of flexion is not recommended, as devices frequently become dislodged, are associated with patient discomfort, and have a higher early risk of failure (Cicolini et al, 2009; Wallis et al, 2014; Larsen et al, 2017; Alexandrou et al 2018). Decisions to insert in areas of flexion, such as the hand or antecubital fossa, are still common in routine clinical practice (Alexandrou, 2019). As evidenced by feedback from clinicians in this study, decisions to insert PIVCs in areas of flexion are often made if no other alternative is available. Consequently, considerations regarding device characteristics that increase the stability, reduce risk of failure, and ensure patient comfort when placed in high-risk areas are still necessary.
Despite the clinical benefits of integrated systems (González López et al, 2014), and the positive attitudes expressed by nurses regarding their use, financial costs associated with these devices should be considered. A recent health economic evaluation found that, despite higher initial per patient cost for an integrated system, the difference in total per patient cost between integrated vs non-integrated systems was similar (ie, US$21.00 v US$20.30) due to the higher risk of unplanned replacements of the non-integrated systems (Tamura et al, 2014). Similarly, a large Australian RCT found that direct hospital costs related to integrated and non-integrated PIVC was approximately AUD $39.00 (AUD $38.10-40.00 or £21.7522.83) per patient (Rickard et al, 2023). Healthcare services should therefore carefully consider how a reduction in device failure, and subsequent re-insertion costs, may offset higher costs of adoption (cost-efficiency).
Previous research establishing the clinical efficacy of integrated PIVCs has highlighted that insertion failure and multiple insertion attempts may demonstrate potential complexity of device insertion or implementation difficulties with unfamiliar devices (González López et al, 2014). The current study found that, despite device complexity, many nurses were positive about integrated PIVCs. However, most nurses included in this study (72%) were not inserters. Recent research has demonstrated comparable first-time insertion success (at least 78%) between integrated and non-integrated devices (Rickard et al, 2023), but further evaluation of the perceptions of nurses credentialed to insert PIVCs, particularly with patients at highest risk of insertion failure or multiple failed insertion attempts, is needed.
Limitations
This study has some limitations. Although designed by clinical experts, the survey was purpose built and did not undergo validity testing. As such, further validation is needed for its use in other settings. Additionally, the survey was disseminated using purposive sampling, direct comparisons between devices were not sought from the same nurse, and may have influenced responses. Each of the nurses surveyed had direct experience with both PIVC systems under evaluation, however, and their responses are reflective of the possible barriers and enablers for implementing these systems into routine clinical practice.
Conclusion
In addition to using best-practice evidence to guide decision-making, healthcare services need to consider the acceptability of the device with end users. By examining the acceptability of these integrated and non-integrated systems as reported by the clinicians who use them, this study was able to provide information on the possible barriers and enablers for implementation of best practice identified by a large RCT. In particular, the integrated system received positive feedback from clinicians who used the device, and has few barriers to its implementation.
KEY POINTS
- Overall, this survey of nurses caring for hospitalised patients with a peripheral intravenous catheter (PIVC) in situ found that the integrated PIVC system received significantly higher positive feedback than the non-integrated standard care product
- Although more skin injuries were identified for the non-integrated PIVC system, participant feedback indicated wings for securement did not guarantee stability for both systems
- In addition to improving clinical outcomes, establishing end-user feasibility and acceptance is important when attempting to change routine clinical practice; however, these must be balanced against any additional associated costs
- Overall, the results of this study complement existing large randomised controlled trials that have found better outcomes associated with using integrated systems
CPD reflective questions
- What are common reasons why peripheral intravenous catheters (PIVCs) fail before completion of therapy?
- What are some important characteristics of PIVCs that influence clinical practice?
- What are some barriers associated with incorporating integrated PIVCs into clinical practice, and how can these barriers be overcome?


