Dear Editor,
We read with interest the article by Marty Cooney et al (2020) that showed significantly reduced central line-associated bloodstream infections after changing to chlorhexidine gluconate/alcohol device swabs instead of alcohol-only swabs in the inpatient dialysis population. Although lowering infections in this patient population is extremely important, the introduction of additional antiseptic agents may increase the risk of adverse events, such as allergic reactions. Our centre has previously reported on the problem of chlorhexidine allergy in patients undergoing urological procedures (Nakonechna et al, 2014; Totty et al, 2017), and a similar trend among dialysis patients has also been reported (Bahal et al, 2017; Chan et al, 2019).
A glimpse of the scale of this problem can be seen in the retrospective survey report of current practice into investigations of perioperative anaphylaxis, with 13 centres (from UK, Europe, Australia, New Zealand and USA) reporting a total of 252 chlorhexidine-related cases of anaphylaxis (Rose et al, 2019), and the 6th National Audit Project of anaesthetic hypersensitivity reactions by the Royal College of Anaesthetists, UK, identified chlorhexidine as the third most common cause of perioperative anaphylaxis (overall 9%) (Cook and Harper, 2018).
Chlorhexidine allergy is now being increasingly recognised to cause a wide spectrum of allergic manifestations, ranging from mild skin reactions to life-threatening anaphylaxis, including in children (Cogné et al, 2017; Egner et al, 2017; Doolan and Crilly, 2019; Opstrup et al, 2019). The recurrent exposure to any substance or chemical compound may lead to IgE-mediated type I hypersensitivity reactions, including anaphylaxis or delayed type IV cellular responses such as eczema, with both types of reactions previously being reported in chlorhexidine-related cases. The clinical severity of such reactions may depend on type of exposure, from localised urticaria to skin cleaning wipes to systemic anaphylaxis seen with chlorhexidine-incorporated indwelling catheters (Nightingale, 1998; Sharp et al, 2016).
Nurses and healthcare assistants should remain vigilant if a patient reports any type of reaction on exposure to chlorhexidine and use the resources of local allergy and immunology services to confirm or refute suspected sensitivity to chlorhexidine. Table 1 shows the different types of chlorhexidine products in use at Hull University Teaching Hospitals NHS Trust and helps identify areas at high risk of allergic reactions. Chlorhexidine is also widely used within many products found in the community, such as antiseptic mouthwashes, toothpastes, sore-throat sprays, powders, creams and contact-lens solutions, which on repeat exposure may serve as a source of primary sensitisation to chlorhexidine.
| At least 14 products containing chlorhexidine in use at the trust contain between 0.015% and 4% chlorhexidine gluconate and are currently being used by at least 44 different areas | ||
| Product type | Product brand (chlorhexidine gluconate) | Number of departments using product |
|---|---|---|
| Surgical scrub | Hydrex 4% | 2 |
| Handrub disinfectant Hibi liquid | 0.5% | 8 |
| Skin-cleansing preparation | Chloraprep Sepp 2% |
44 |
| Antiseptic cleansing solution | Unisept 0.05% |
3 |
| Wound dressing | Bactigras 0.5% |
8 |
| Antimicrobial skin cleanser | Hibiscrub 4% | 4 |
| Antimicrobial lotion 0.9% | Skinsan 0.9% | 3 |
| Skin preparation with coloured tint | Chloraprep tint 2% | 14 |
| Lubricant containing lidocaine | Instillagel 0.25% | 29 |
| Dry shampoo cap | Clinell shampoo cap 2% | 2 |
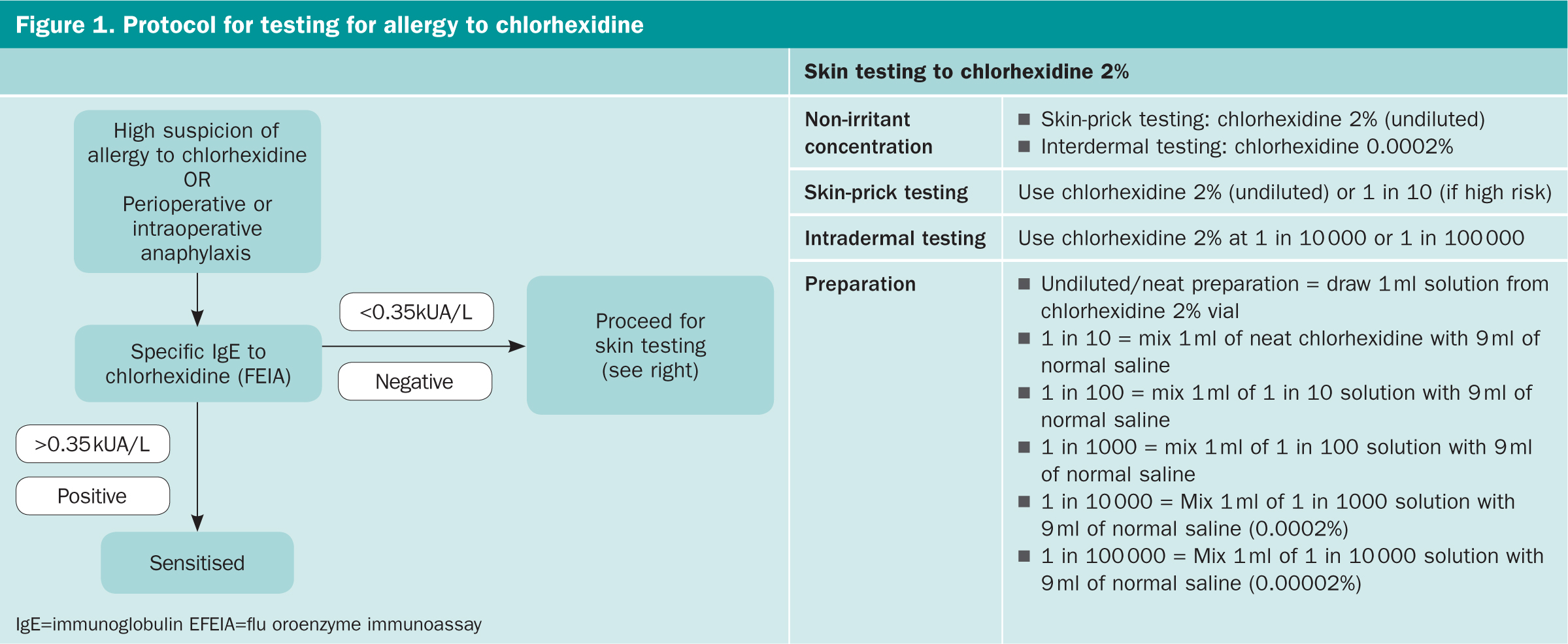
Skin tests
To investigate cases of suspected allergy to chlorhexidine, we routinely measure specific IgE antibodies to chlorhexidine and perform skin tests (Figure 1). Our local laboratory records for the past 2 years (2018–2019) showed a total of 109 requests for specific-IgE antibodies to chlorhexidine for seven patients (6.4%) found to be positive (up to 4.6 AU/ml, ImmunoCAP) at the cut-off of 0.35 AU/mL and 16 (15%), if the cut-off was lowered to 0.10 AU/ml.
A retrospective analysis of 25 positive drug tests out of a total of 186 tests performed at our centre in the past 2 years (2018-2019) identified two patients (8%) with confirmed allergy to chlorhexidine.
The first patient was a 47-year-old man who had anaphylaxis during angioplasty with hypotension (60/30 mmHg) and tachycardia requiring resuscitation. Tryptase levels spiked to 26.7 ng/ml that normalised (4.6 ng/ml; normal range, 2-14 ng/ml) by the next day. Chlorhexidine-specific IgE was only 0.22 AU/ml (<0.35 AU/ml), but a skin test was positive and the rest of the drugs tested (anaesthetic agents, iodine dyes, latex) were negative. He had undergone two angioplasties 3 months and three years before this event and on both occasions had developed urticaria.
The second patient was a 33-year-old woman who underwent multiple procedures under anaesthesia, including gynaecology, obstetrics, urology and colorectal surgery, but who subsequently developed significant hypotension on induction of anaesthesia during replacement of ureteric stent requiring cardiopulmonary resuscitation for PEA [pulseless electrical activity] cardiac arrest. She was successfully resuscitated with intravenous adrenaline, leading to full recovery. Tryptase taken at 4 hours was significantly raised at 31.3 ng/ml. There were detectable specific-IgE antibodies to chlorhexidine (2.0 AU/ml) with positive skin tests to mivacurium and atracurium. The near fatal event is probably explained by sensitisation to both chlorhexidine and neuromuscular blocking agent in this patient.
The numbers of referrals for allergy testing and sensitisation rates indicate that chlorhexidine allergy is likely to reach similar proportions as latex allergy did in the 1990s (Laxenaire, 1999). Furthermore, patients on dialysis are frequently on medications to suppress uraemic pruritus, and it is therefore likely that some patients will experience multiple milder reactions before chlorhexidine allergy is suspected.
In conclusion, although use of chlorhexidine-incorporated skin swabs may lower infection rates, it is not without an added risk of adverse allergic reactions, which can be reduced by increasing awareness of chlorhexidine allergy among healthcare practitioners, patients and their carers.

