The lymphatic system has three major roles in human physiology: regulation of homeostasis, removal of fat from the gut to the blood stream and immune trafficking (Jiang et al, 2019). Lymphoedema is associated with dysfunctional lymphatics, tissue fibrosis and inflammatory changes in the skin and local tissue (García Nores et al, 2018). Dunnill et al (2017) highlighted that poor tissue perfusion and oxygenation are major factors in the sequence leading to epithelial damage in wound formation. They also acknowledged that oxygen was an essential substrate required to supply the amount of energy required for tissue renewal (García Nores et al, 2018). Providing patients with safe equipment such as compression which, in turn, also enhances their tissue health, is paramount when supporting self-management regimens. Ensuring that compression supports tissue health is crucial to the management of lymphoedema (Wanchai et al, 2016). In a 24-hour management plan, a range of compression devices should be available to meet individual needs through the day, without compromising their lymphoedema and tissue health during activity change (Whitaker, 2016; Bertsch, 2018).
If left untreated, lymphoedema can develop into different stages of disease progression as has been documented by the International Society of Lymphology (ISL) in the summarised stages 0-3 (ISL, 2016). As lymphoedema progresses through these stages, increased changes in tissue fibrosis and adipose tissue can be observed, replacing the once ‘pitting oedema’ tissue (ISL, 2016). An increase in obesity in western cultures has become a global issue and the World Health Organization (WHO) (2018) recorded that, in 2016, more than 1.9 billion people aged 18 years and over were overweight, with more than 650 million considered to be obese. Moreover, the adult obesity rates were highest among the region of the Americas (29%), Europe (23%) and Eastern Mediterranean (21%) (WHO, 2018). The expansion of adipose tissue in obesity has been linked to an inappropriate supply of oxygen and hypoxia development, which then further initiates adipose tissue fibrosis (Buechler, 2015) and, in turn, is aggravated further by inflammation. Adipose tissue, fibrosis and inflammation are all common presentations in later stages of lymphoedema (ISL, 2016). Dysfunctional lymphatic systems sensitise individuals to become obese, and obesity can worsen lymphatic function (Jiang et al, 2019).
Deteriorating lymphoedema, as well as other factors, may result in an increase in localised adipose tissue and obesity, thus causing tissue inflammation such as that seen in venous disease. Venous insufficiency may therefore lead to a situation of sustained cellular hypoxia (Ortega et al, 2018). This implies that, clinically, the condition may continue to deteriorate where the environment is not supported or corrected. Many patients who have chronic oedema have a venous component to their condition and, in western countries, this affects up to 80% of the adult population, suggesting that the presence of venous insufficiency may contribute to further deterioration due to hypoxia (Ortega et al, 2021).
A genetic study looking at the autosomal dominant condition primary congenital lymphoedema Milroy's disease, reviewed the clinical findings of 71 patients (Brice et al, 2005). Not unexpectedly 90% had presence of oedema, however more surprisingly 23% had large calibre leg veins. Primary lymphoedema is predominantly associated with failure of the lymphatics, however this study (Brice et al, 2005) showed that other vascular structures can also be affected, leading to the consideration of management strategies. Both lymphatic and venous disorders are managed primarily through conservative approaches using compression, such as bandages, stockings and wrap systems (Felty and Rooke, 2005; Kalodiki et al, 2007; Cooper, 2015; Green 2018; Chiang et al, 2019).
Night-time compression remains an area that is gaining momentum in the literature. A study across five countries showed that up to 89% of individual with chronic swelling observed an increase in volume overnight, if no compression is used (Whitaker, 2016). This is contrary to the suggestion that limb swelling will reduce overnight or stay the same due to elevation (Bertsch, 2018). A new night-time compression device has recently been shown to improve tissue oxygenation levels in healthy legs. This study suggested that this new device may make night-time compression more tolerable for people with oedema (Chohan et al, 2019). As only healthy individuals with no oedema were used in this previous study, it was not possible to undertake limb volume measurements. The case study presented in this article explores the short-term impact of using a night-time compression garment for primary lymphoedema on tissue oxygen and limb volume.
Methods
Patient history
A single female patient aged 18 years, with primary lymphoedema (Milroy's disease), was recruited to the study. She was diagnosed at birth and followed up in a genetics centre. The family history of the patient was mapped back to her great grandfather from a symptoms perspective. From a genetic perspective, patient history was traced back to her mother and aunts. The primary lymphoedema was also associated with venous prominence with large veins and was visible bilaterally (Figure 1a).
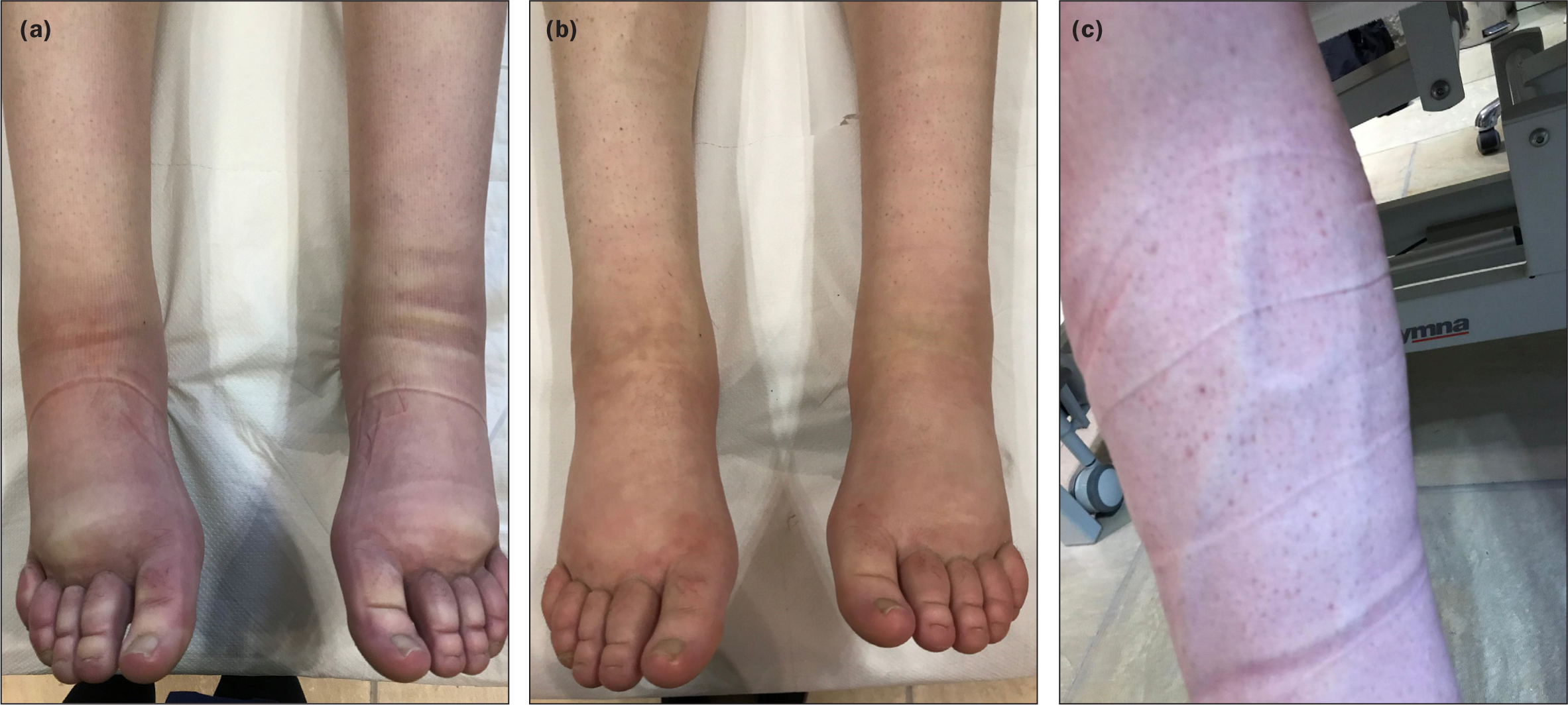
Management since birth had been initially skin care and self-massage, then during the patient's adolescence compression garments were introduced. These consisted of toe garments and flat-knit, made-to-measure, below-knee compression stockings, 20-30 mmHg. Five weeks prior to the study, the patient underwent a short course of three treatments in one week, involving:
- Decongestive lymphoedema therapy (DLT)
- Negative pressure lymph drainage (NPLD)
- Multi-layer lymphoedema bandaging (MLLB).
Following treatment the patient was fitted with new, made-to-measure garments as previously stated. All data collection conformed to the Declaration of Helsinki and the General Data Protection Regulations (GDPR), and the patient provided informed written consent prior to participation.
Procedure
The patient visited the testing facility for two 1-hour sessions, 1 week apart. At the first session, baseline anthropometric measures were taken from the right lower leg to enable sizing in line with current manufacturer guidance. As previous research has shown that there is no significant difference in tissue oxygenation levels between long sitting (sitting up with a supported back and legs horizontal) and lying supine in healthy legs, lying supine was omitted from this study (Chohan et al, 2019). The participant was then tested in one of two sitting positions—chair-sitting and long sitting—in a randomised order (www.randomization.com). Skin temperature was taken from the calf at baseline followed by measurements at 20 min, 40 min and 60 min.
Tissue oxygenation (StO2) was measured throughout each session using a near-infrared spectroscopy monitor (MoXY Monitor, MN), averaging tissue oxygenation over each of the three 20-minute periods (baseline, during intervention, and post-intervention). The intervention consisted of the application of a compression garment (TributeWrapTM, Lohman-Rauscher, DE) (Figure 2) to the right leg following the 20-minute acclimatisation period. The participant completed a series of questions following each session relating to comfort, sensation and potential adherence to the intervention if it was to be prescribed by a clinician.
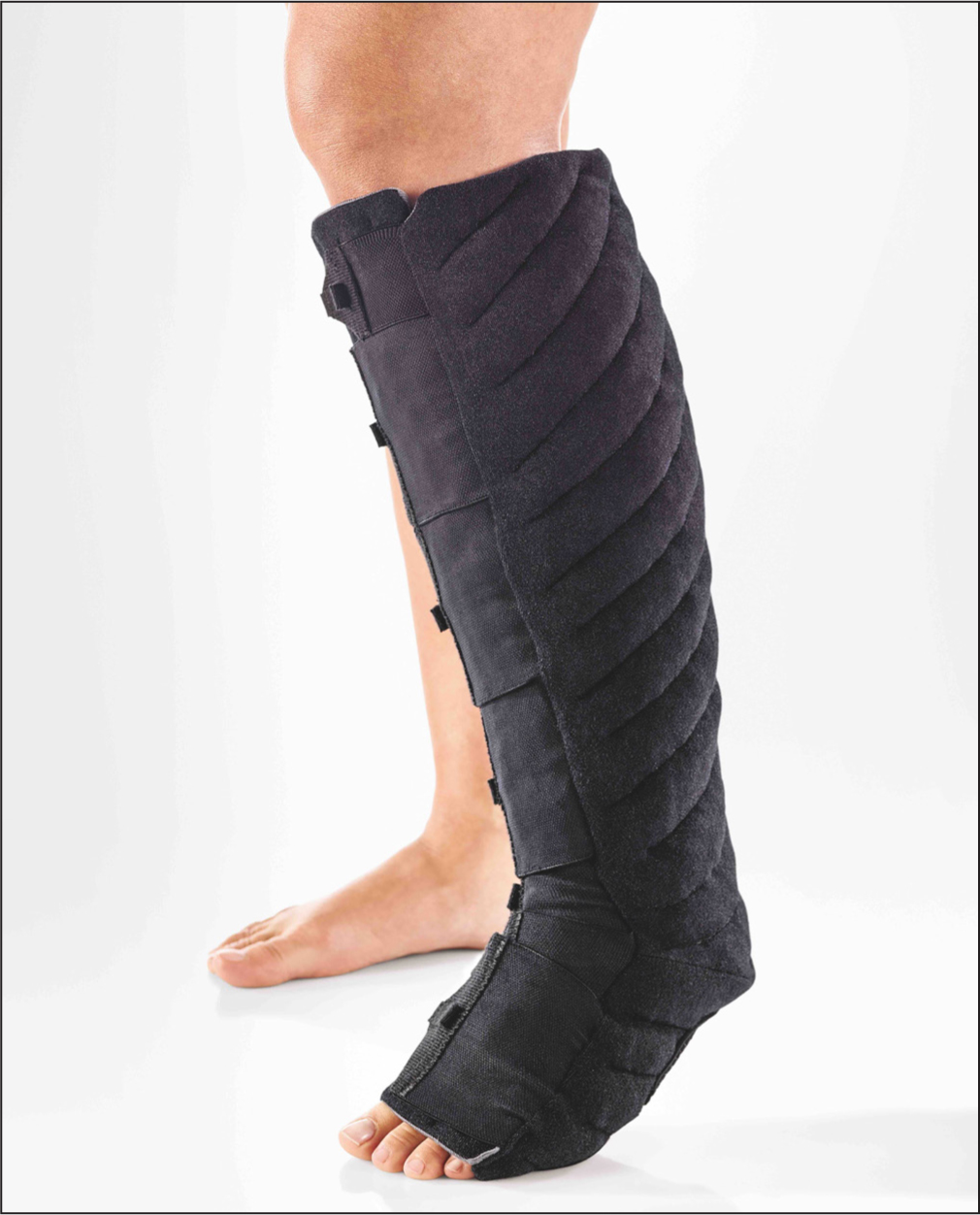
Limb volumes, using the 4 cm pre-tension tape measure method (Williams and Whitaker, 2015), were recorded on both the right and left leg to knee level, pre- and post-long-sitting intervention.
Data analysis
Percentage change in tissue oxygenation was calculated in each position. Change in millilitres was noted in limb volumes of both legs, using the frustum formula (using a 4 cm truncated cone) (Sitzia, 1995). Analysis of the limb volume method was done using the percentage change in excess and absolute limb volume over time (Williams and Whitaker, 2019).
Results
Position, temperature and tissue oxygenation
Change in tissue oxygenation was monitored pre, during and post the 20-minute intervention (Figure 3 and Table 1). In the long-sitting position, StO2 levels started high at baseline (94.5%), remaining relatively maintained both during and post a short 20-minute intervention (94.1%). In the chair-sitting position, StO2 levels were significantly lower at baseline (52%), increasing by 77% to 92% tissue oxygenation during the intervention, followed by a 9% drop post-intervention to 83.7%.
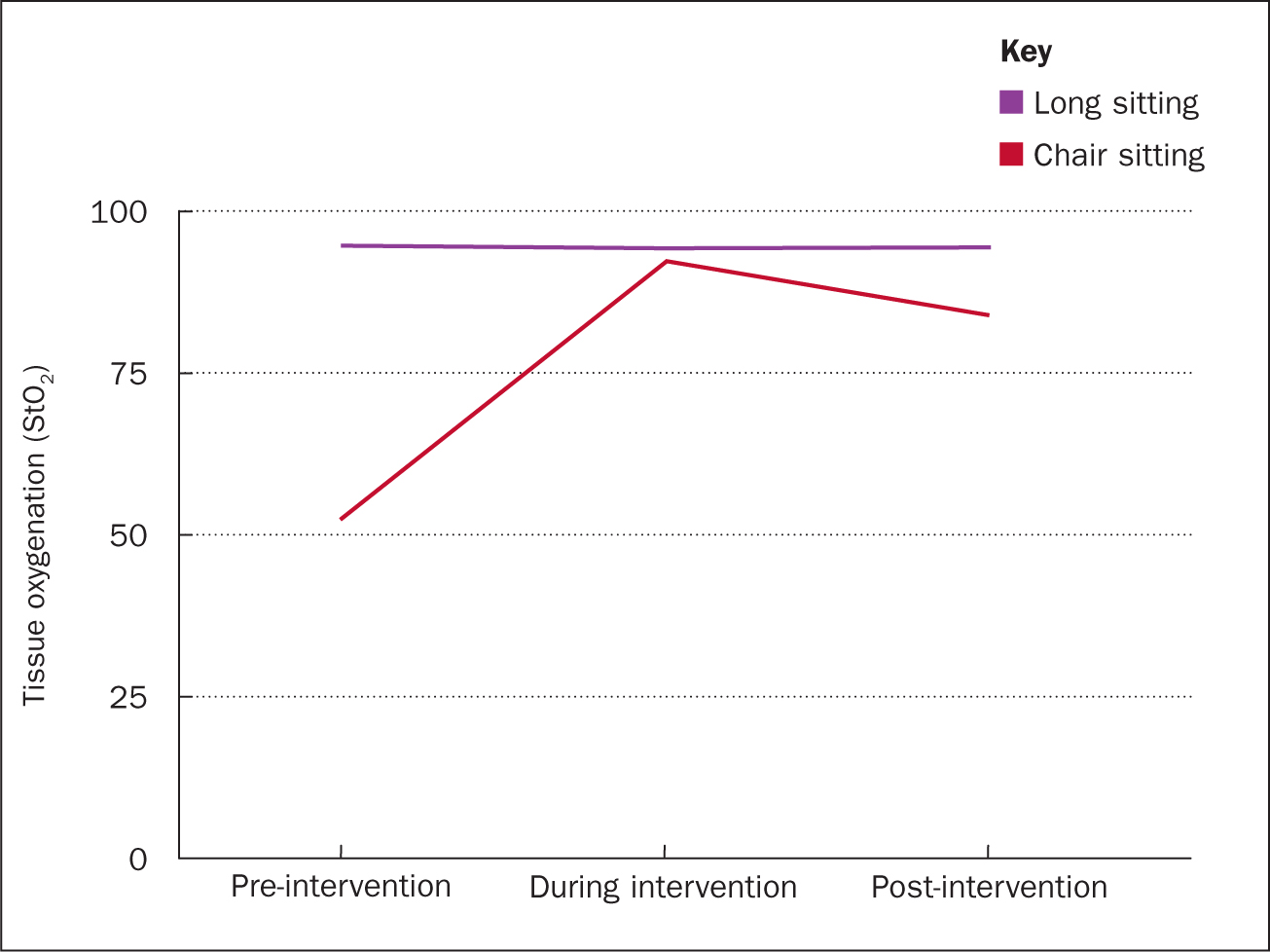
Table 1. Tissue oxygenation (StO2) and temperature data
| Chair | Long sitting | |
|---|---|---|
| Raw data (StO2) (%) | ||
| Baseline (pre-intervention) (20 minutes) | 51.98 | 94.47 |
| During intervention (20 minutes) | 92 | 94.11 |
| Post-intervention | 83.73 | 94.18 |
| Percentage change in StO2 | ||
| Measurement at baseline compared with during intervention | 77.00 | -0.38 |
| Measurement during intervention compared with post-intervention | -8.99 | 0.07 |
| Overall change (measurement at baseline compared with post-intervention) | 61.09 | -0.31 |
| Raw data (temperature °C) | ||
| Baseline (20 minutes) | 36.3 | 36.6 |
| During intervention (20 minutes) | 36.5 | 37 |
| Post-intervention (20 minutes) | 36.3 | 37.6 |
It is known that at least a 0.5°C change is needed in temperature to be clinically important. While in the chair sitting position there was no clinically important change in temperature, it is important to note that in the long sitting position there was a 1°C change in skin temperature at the calf.
Limb volume
Throughout the treatment protocol, the patient removed the regular compression following several hours of wear and proceeded to the long-sitting position, so she was without compression for a period of just 20 minutes prior to the sleeve being applied to the right leg for 20 minutes.
Limb volume of the right leg pre-intervention was 2509 mL and of the left leg 2529 mL. Post-intervention the right leg measured 2444 mL and left leg 2530 mL, with a total reduction of 65 mL in the right leg and an increase of 1 mL in the left.
Patient feedback
The patient described comfort levels highly positively in both positions, with further comparisons made to the reduction in swelling seen after bandaging, and also comparing the effects with the wear of hosiery, with more movement possible reported by the patient post-intervention.
The patient reported that compliance was extremely likely due to comfort and the ease of application.
Discussion
All interventions occurred upon removal of the patient's standard compression garments (20-30 mmHg). Because this was her daily routine, it was agreed to use this schedule. In a previous study that measured tissue oxygenation in healthy legs using this type of compression (Chohan et al, 2019), an average for 28 participants' oxygen level baseline for healthy legs without any compression in the chair-sitting position was 56%.
In the case study described in this article, the patient's tissue oxygenation level on immediate removal of compression was 52%. Although the patient's oxygenation level was lower than the average of 28 healthy participants reported in Chohan et al's study (2019), the difference was only by 4%, indicating that the flat-knit 20-30 mmHg compression garments maintain tissue oxygen levels similar to those in healthy individuals with no compression.
When considering the patient's StO2 in the long-sitting intervention this started and was maintained at a higher oxygenation level (94%), supporting the clinical advice to elevate the limbs when at rest. Compared with the control limb (left leg) that remained unchanged, the limb volumes decreased in the intervention limb (right leg). This small decrease of 65 mL in only 1 hour of positioning and 20 minutes of intervention using the compression garment indicated that the product's use of foam chips and sewn chevron design that mimic the lymph pathways may have contributed to the maintenance of the StO2 level and decrease in limb volume. It also supports the maintenance of limb volume on the left leg with no intervention on leg elevation.
At the end of a long day when a patient removes their standard compression for the night, a limb can swell once the garment has been removed, however this is minimal or static when the limb is elevated, as shown with the patient's left leg in this case study. Therefore, rather than simply maintaining a static position with no compression, this intervention may be a suitable option for a normal night-time management regimen for both limbs. Although there was a small change in volume during the short intervention reported in this case study, the patient's limb volume did reduce while wearing the compression sleeve.
The chair-sitting position provides good insight into the benefits of wearing compression garments that use alternative materials while remaining in a static position and posture for 1 hour, during which there was a 20-minute intervention using a compression sleeve. The 77% increase in StO2 levels to 92% showed significant improvement. This provided a key insight into understanding that different product options can produce results akin to specific positioning of limbs, eg elevation of legs. This garment offers patients and professionals a choice when considering individual patient lifestyles, activities and needs, supported by understanding that good tissue health can be maintained through a non-dynamic intervention; this option can also improve concordance.
The participant reported that comfort levels and the ease of application were attributes that would enhance her self-management regimen. Having a choice of different compression appliances throughout a 24-hour period, which is of benefit to tissue health, should be available for all patients.
Conclusion
Individuals cannot be mobile for entire 24-hour periods. To ensure better self-management, appliances that not only improve tissue health in a chronic disease setting, but that enable tissue health to be maintained upon removal, should be priority when considering a care regimen for people with lymphoedema. Knowing the disease progression pathway of lymphoedema (ISL, 2016), maintaining a healthy tissue environment can ensure that deterioration and further progression of the disease are kept to a minimum.
The complexities of tissue hypoxia may lead to deterioration of the superficial tissues, delay wound healing when present, encourage inflammation and then, conversely, development of localised adipose tissue. Making available a selection of appliance types encourages patients to self-manage their condition because they can be secure in the knowledge that the garments will increase tissue oxygen levels, and this can only enhance control of patients' disorder and encourage self-care.
KEY POINTS
- Use of the night-time compression garment used in this study maintained the effects of the patient's compression stockings in the long-sitting position
- In the chair-sitting position, there was a 77% increase in tissue oxygenation through a short intervention
- Clinicians should consider offering patients a wider garment range of garments and encourage the use of night-time compression as part of a 24-hour management plan
CPD reflective questions
- Reflect on why self-care important for individuals with Milroy's disease
- Think about why it is important to give patients a choice of products for 24 hour management
- Consider how the available selection of night-time compression garments could affect concordance and impact on patient outcomes


