Under-nutrition, which is a major form of malnutrition, is associated with improper balance between caloric delivery and caloric needs (Lew et al, 2017; Al-Kalaldeh et al, 2018). Critically ill patients, including those with severe traumatic brain injury (TBI) and acute stroke, are at risk of nutritional failure due to hypermetabolism and gut dysfunction, and nutritional failure leads to poor prognosis (Chapple et al, 2015; McClave et al, 2016; Yuan et al, 2019). This is because nutritional failure results in delayed immunity, delayed recovery, and increased mortality in addition to challenges in the weaning process from mechanical ventilation (Btaiche et al, 2010; McClave et al, 2016; Yuan et al, 2019).
Despite its physiological superiority over parenteral nutrition, enteral nutrition (EN) is still connected with a range of feeding-related complications. These include, but are not limited to, aspiration, pneumonia, feeding intolerance, re-feeding syndrome, diarrhoea and hyperglycaemia (McDermid et al, 2010; Kalaldeh and Shahin, 2015). The association between feeding-related complications and inadequate caloric attainment and nutritional failure is well established (Heighes et al, 2010; Kalaiselvan et al, 2017). Practices recognised as triggering nutritional failure in the intensive care unit (ICU) include non-systematic management of gastric residual volume (GRV), poor implementation of aspiration reduction measures (ie, detecting inadvertent tube displacement, and using prokinetic agents), and frequent unnecessary feeding interruption (Cahill et al, 2014; Dhaliwal et al, 2014). Focused assessment aims to maintain ongoing monitoring for certain nutritional outcomes to enhance nutritional adequacy and caloric attainments, and detect feeding-related complications early once EN is initiated (Heighes et al, 2010; McClave et al, 2016).
An international observational study conducted on TBI patients in ICU found that only 58% of them received the estimated required energy intake, and those who did not had a longer hospital stay (Chapple et al, 2015). Another retrospective observational study suggested that patients with severe TBI admitted to the ICU had a higher survival rate in the first 30 days due to successful EN administration (Papadimitriou-Olivgeris et al, 2021). Regarding severe acute stroke patients, evidence affirmed that the risk for malnutrition among these patients in the ICU is still underestimated (FOOD Trial Collaboration, 2003; Sremanakova et al, 2019; Yuan et al, 2019). For instance, a study by Sremanakova et al (2019) reported a reduction of body weight of more than 3 kg in a short-term period for patients with acute brain attack, which in turn increased the mortality rate. Thus, ICU patients with severe brain attack (TBI or acute stroke) required compensatory nutritional support to accommodate hypermetabolic state and hypoproteinaemia (Wang et al, 2013; Chapple et al, 2016).
Aim
This study aimed to estimate the time point where the development of nutritional failure and feeding-related complications are more likely among critically ill, mechanically ventilated patients following severe brain attack, including TBI and acute stroke.
Methods
This prospective observational study took place in six ICUs from two main governmental referral medical centres in Jordan. The aim of the study was achieved through determining eligible patients for bedside observation and then performing data observation for predetermined parameters at five successive points over 9 days on an every-other-day basis, starting from EN initiation. The initial assessment of the selected medical centres showed that none of them had adopted an exhaustive evidence-based protocol for EN administration. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Lachat et al, 2016) were followed.
Sampling
The study specifically observed patients who suffered from either TBI or acute stroke (cerebrovascular accident) who were admitted to the ICU after a significant deterioration in the level of consciousness and the need for mechanical ventilation. The patients selected for bedside observation were adult patients aged 20–60 years who were fed enterally and mechanically ventilated. Observation included only the newly admitted patients who had not already started on EN. Therefore, patients who started with EN before the data-collection period were excluded even if they were otherwise eligible for participation. This is because the aim of the study was to commence observation from the zero point of starting EN administration.
Patients known with comorbidities such as diabetes mellitus, heart failure, chronic kidney disease, chronic liver disorders, and immunocompromised patients were excluded from selection. Patients were also excluded from if they were fed enterally in combination with parenteral nutrition. In addition, severely haemodynamically unstable status, which was defined as refractory hypotension that required higher doses of inotropic agents—such as more than 25 µg/kg/minute for dobamine and dobutamine, over 0.5 µg/kg/minute for adrenaline (epinephrine) and noradrenaline (norepinephrine), or over 0.04 units/minute for vasopressin—were also excluded.
Sample size was estimated using the following formula:
n = z 2 p q / d 2
Where n: sample size; z: Z-score of standard deviation which is equal to 1.96; p: proportion; q: 1-p; d: α (Daniel, 2009).
The global prevalence of severely injured brain attack patients has been estimated at 60 cases per 100 000 (Dewan et al, 2018). Using this prevalence, the calculation of the sample size revealed the required sample size was 86 subjects.
Bedside observation
The study retrieved patients' admission parameters from the electronic data system, which included general demographic data and assessment data required to calculate the APACHE II score (Acute Physiology and Chronic Health Evaluation II) (Knauss et al, 1985). The five-point bedside observation included the following clinical nutritional outcomes:
- Measurement of GRV
- Measurement of body weight
- Measurement of serum albumin
- Calculation of the caloric attainment
- Calculation of the Malnutrition Universal Screening Tool (MUST) score (assessed 3 times over the 9 days)
- Detection of feeding-related complication, which included: pulmonary aspiration, pneumonia, diarrhoea, feeding intolerance, re-feeding syndrome, and hyperglycaemia.
MUST was used to estimate the risk of malnutrition through measuring anthropometric characteristics such as height, weight, body mass index (BMI), and weight change over time. Using alternative scales such as length of forearm (ulna) and knee height to measure height, and mid upper-arm circumference to estimate a BMI were considered when usual height and weight could not be obtained. MUST scores provided inferences of malnutrition at three levels: 0=low risk, 1=medium risk, and 2 or more=high risk (Todorovic et al, 2016).
Energy requirement was adjusted at 25 kilocalories per kilogram of actual body weight (25 kcal/kg) per day (Harvey et al, 2014) unless medical orders differed for specific patients. Dietitians had contributed to the calculation of caloric attainment in every observation day. In general, energy deficit was calculated based on kilocalories per day. Protein intake was recorded on daily observation measurements, in grams per day, but protein deficit was not calculated. However, dietitians assumed that protein intake less than 1 g/kg/day was the same as caloric deficit and marked ‘No’ in the caloric attainment section.
Intensivists had assisted in confirming the diagnosis of some feeding complications such as pulmonary aspiration, pneumonia, feeding intolerance, and re-feeding syndrome. For instance, feeding intolerance was diagnosed when the following signs were reported simultaneously: vomiting, nausea, abdominal pain and/or distension, constipation, and diarrhoea (Moore et al, 2003; Binnekade et al, 2005; Btaiche et al, 2010). Diarrhoea was defined as at least three liquid stools observed in a day, excluding the effect of laxative agents or infections caused by Clostridium difficile (Thibault et al, 2013). Hyperglycaemia was detected when a serum glucose test exceeded 180 mg/dL for non-diabetic patients (Harvey et al, 2014).
Data collection
Ethical approval to undertake the study was obtained from an institutional review board (No: 11349/2018). Data collection extended from April 2019 to March 2020. Bedside observation was performed directly by arrangement between the researchers and the nurses in charge of each ICU, who added additional task to the daily duties to identify eligible patients admitted to the unit. An introductory session was provided for each of the six ICUs, for all staff. At the session the study was explained and the contact details of the principle investigator (PI) were provided. Once a patient who met the inclusion criteria was admitted to the unit, the researcher was contacted on the same or next day to confirm the patient's admission and request to be notified when EN was initiated. Thereafter, the researcher provided the in-charge and assigned nurses with the information sheet, which needed to be filled out with specific data on a scheduled observation on the first, third, fifth, seventh and ninth days once EN was initiated. It was a responsibility of the PI to keep in close contact with the nurses, dietitian on duty and resident intensivist to report observation parameters precisely. As an attempt to validate observation data, the PI performed inter-observer agreement for the recorded data for some randomly selected patients. Regular visits by the PI aimed also to follow up for unexpected observations such when it needed to be terminated due to physiological deterioration, transfer, discharge or death.
Statistical analysis
Data were entered into SPSSv 19.0 software. Descriptive statistics included frequency, percent, mean, standard deviation (SD), median, and inter-quartile range (IQR). Differences between observations was tested by chi-square test for categorical variables and Paired-Samples T test for continuous variables. Multivariate analysis was used to assess the relationship between significantly correlated variables (factors) and caloric attainment using binary logistic regression. The regression model included the 95% confidence intervals (CI).
Results
A total of 84 patients were initially selected for bedside observation. On the final day of observation, 63 patients were successfully observed, meaning that 25% of the initial sample were not included due to deterioration, transfer, discharge or death. The median of patients' ages was 53 years old and the majority (76%) were male (Table 1). Patients were distributed roughly equally between acute brain stroke and TBIs. The estimation of mortality at ICU admission indicated a 55% average mortality rate using the APACHE II score (non-operative estimate), which scored a mean of 26.7. The median length of using mechanical ventilation was 7 days, whereas EN was initiated on average on the fourth day of ICU admission. More than half of the patients (57%) were on intermittent feeding methods and about half of them received a prokinetic agent.
Table 1. Patients' demographics and admission criteria (n=84 patients)
| Variable | Category | Value |
|---|---|---|
| Age (years), median (IQR) | 53 (35.7–63.8) | |
| Sex, n (%) | Male | 64 (76.2%) |
| Female | 20 (24%) | |
| Admission diagnosis, n (%) | Acute stroke | 40 (48%) |
| TBI | 44 (52%) | |
| APACHE II score, mean (SD) | 26.7 (7.3) | |
| Length of MV/days, median (IQR) | 7 (2-12) | |
| Day starting EN since admission, median (IQR) | Day 4 (2–5) | |
| Use of prokinetic agents, n (%) | Yes | 44 (52%) |
| No | 40 (48%) | |
| Method of EN, n (%) | Intermittent | 48 (57%) |
| Continuous | 36 (43%) |
APACHE=Acute Physiology and Chronic Health Evaluation; EN=enteral nutrition; IQR=interquartile range; MV=mechanical ventilation; SD=standard deviation; TBI=Traumatic brain injury; n=number
Assessment of nutritional outcomes
The nutritional outcomes over the five points of assessment are presented in Table 2. Starting with the GRV, mean volumes increased constantly from day 1 to day 9. Figure 1 shows that a sharp significant increase in the GRV was evident between day 3 and day 5 (P=0.029). Regarding body weight, results showed a constant reduction in patients' body weight between day 1 and day 9. Figure 2 illustrates that the most significant notable reduction was reported between day 3 and day 5 followed by a significant drop after day 7 (P=0.015 and P<0.001, respectively). Likewise, serum albumin level constantly decreased between the first and the last observation points.
Table 2. Clinical nutritional outcomes (n=84)
| Variable | Day 1 | Day 3 | Day 5 | Day 7 | Day 9 | P value | |
|---|---|---|---|---|---|---|---|
| n=84 | n=77 | n=74 | n=67 | n=63 | |||
| GRV (ml), mean (SD) | 144 (16.4) | 126 (14.1) | 142 (15.6) | 173 (18.5) | 196 (20.4) | <0.001 | |
| Body weight (kg), mean (SD) | 79.4 (23.5) | 78.7 (21.8) | 77.4 (16.2) | 77.1 (15.7) | 74.3 (17.7) | <0.001 | |
| Serum albumin g/dL, mean (SD) | 3.6 (0.5) | 3.5 (0.5) | 3.3 (0.6) | 3.2 (0.6) | 3.1 (0.7) | <0.001 | |
| MUST score, n (%) | Low risk | 46 (55%) | 26 (35%) | 16 (25%) | 0.03 | ||
| Medium risk | 22 (26%) | 32 (43%) | 25 (40%) | ||||
| High risk | 16 (19%) | 16 (22%) | 22 (35%) | ||||
| Patients who attained the caloric requirement, n (%) | 51 (61%) | 43 (56%) | 28 (38%) | 19 (28%) | 18 (29%) | <0.001 | |
GRV=gastric residual volume; kg=kilogram, MUST=Malnutrition Universal Screening Tool; SD: standard deviation
P value, which represents differences in the values between observations, was tested by chi-square test for categorical variable and paired-samples T test for continuous variables
Figure 1. Changes in the GRV across observation period
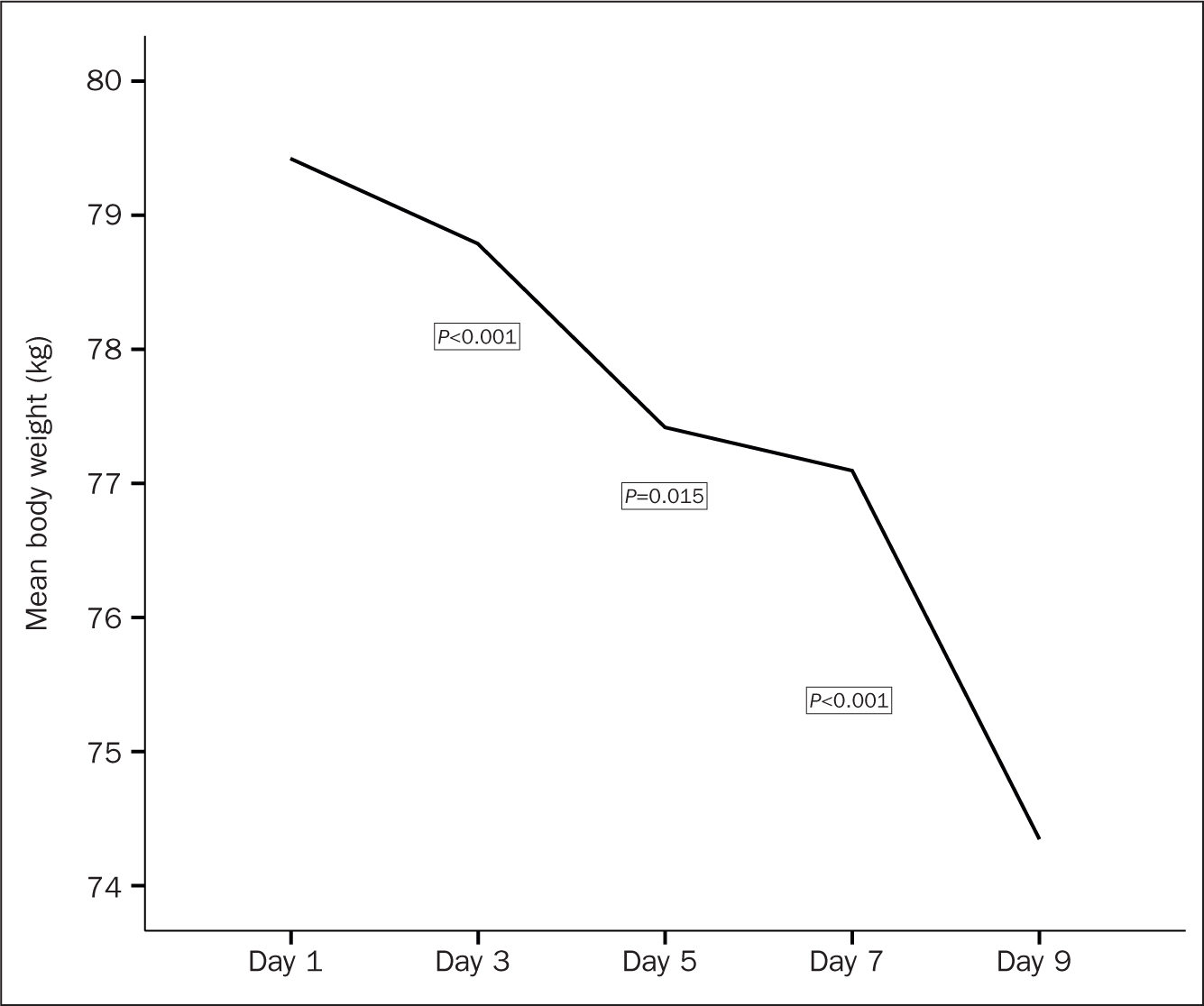 Figure 2. Change in body weight across observation period
Figure 2. Change in body weight across observation period
In respect of the screening for malnutrition using the MUST score, there was a steady decrease in the number of patients who revealed low risk of malnutrition alongside a steady increase in the number of patients who were deemed to be at high risk of malnutrition throughout the observation period (Table 2). These results were congruent with the determination of caloric attainment for observed patients between day 1 and day 9. Caloric attainment was achieved in 61% of patients after the first day of EN administration compared with only 29% of patients at day 9. It was noted that the sharpest drop in caloric attainment occurred between day 3 and day 5 of observation (56% vs 38%, respectively).
Incidence of feeding-related complications
As mentioned, the detection of feeding-related complications commenced from the first day of observation as shown in Table 3. Feeding intolerance gained the highest incidence from the first day of observation and remained high until day 9 (42% and 51%, respectively). The peak of incidence of feeding intolerance was at day 7 (61%). Pulmonary aspiration was reported in 10% of patients at the first day of observation to reach the peak of incidences at day 5 (34%) then reduced to 24% at day 9. Pneumonia and diarrhoea exhibited nearly the same pattern of incidence in which both had reached the highest rates at day 5 (24% and 36%, respectively). Although hyperglycaemia was reported less often, the peak of its incidence was reported at day 3 (16%), but it was subsequently returned to its low rate at day 9. Although re-feeding syndrome was not reported at the first 3 days of observation, it developed in a few cases starting from day 5.
Table 3. Feeding-related complications (n=84)
| Variable | Day 1 | Day 3 | Day 5 | Day 7 | Day 9 | P value* |
|---|---|---|---|---|---|---|
| n=84 | n=77 | n=74 | n=67 | n=63 | ||
| Aspiration, n (%) | 8 (10%) | 12 (16%) | 25 (34%) | 20 (30%) | 15 (24%) | <0.001 |
| Pneumonia, n (%) | 7 (8%) | 10 (13%) | 18 (24%) | 13 (19%) | 16 (25%) | <0.001 |
| Diarrhoea, n (%) | 18 (21%) | 24 (31%) | 27 (36%) | 23 (34%) | 21 (33%) | <0.001 |
| Feeding intolerance, n (%) | 35 (42%) | 29 (38%) | 36 (49%) | 41 (61%) | 32 (51%) | <0.001 |
| Re-feeding syndrome, n (%) | 0 (0%) | 0 (0%) | 6 (8%) | 8 (12%) | 8 (13%) | 0.001 |
| Hyperglycaemia, n (%) | 8 (10%) | 12 (16%) | 5 (7%) | 3 (4%) | 6 (10%) | <0.001 |
Factors associated with nutritional deficit
Binary logistic regression was employed to examine factors associated with low caloric attainment. In the Hosmer-Lemeshow goodness-of-fit test, poor fit was manifest in the chi-square value of 10.708 and 8 degrees of freedom at a significance level of 0.219.
The model revealed six significant variables (time since EN initiation P<0.001, age P<0.001, length of mechanical ventilation P=0.048, GRV P<0.001, diarrhoea P=0.010, and feeding intolerance P<0.001) (Table 4). All these factors appeared to influence the caloric deficit. Specifically, older patients with longer dependence on mechanical ventilation and higher GRV values, in addition to the incidence of diarrhoea and feeding intolerance, are more likely to develop nutritional failure especially after day 3 of EN initiation (Table 4).
Table 4. Regression model of enteral nutrition caloric attainment
| Variable | B | SE | Wald | df | P value | 95% CI |
|---|---|---|---|---|---|---|
| Time since EN initiation | -0.386 | 0.074 | 26.992 | 1 | <0.001 | 1.272 – 1.701 |
| Age | -0.070 | 0.032 | 4.865 | 1 | 0.027 | 0.876 – 0.992 |
| APACHE II score | -0.053 | 0.070 | 0.559 | 1 | 0.455 | 0.918 – 1.210 |
| Duration of MV | -0.134 | 0.068 | 3.909 | 1 | 0.048 | 1.001 – 1.306 |
| GRV | -0.003 | 0.001 | 11.685 | 1 | 0.001 | 1.001 – 1.004 |
| Aspiration | -0.186 | 0.283 | 0.435 | 1 | 0.510 | 0.477–1.445 |
| Diarrhoea | -0.658 | 0.254 | 6.697 | 1 | 0.010 | 1.173 – 3.178 |
| Feeding intolerance | -1.014 | 0.252 | 16.210 | 1 | <0.001 | 1.683 – 4.519 |
APACHE=Acute Physiology and Chronic Health Evaluation; B=provides estimate for the mean difference in EN caloric attainment; CI=confidence interval; df=degree of freedom; EN=enteral nutrition; GRV=gastric residual volume; MV=mechanical ventilation; SE=standard error; Wald=used to test the true value of each variable based on the sample estimate
Discussion
Despite nutritional support initiated within the early days of ICU admission, this study confirms the development of malnutrition among critically ill patients who suffered from brain attacks. Failure to attain caloric requirements was associated with a rapid significant reduction in body weight, specifically, between the third and fifth day of observation. Similarly, a steady reduction in serum albumin was also evident throughout the 9-day observation. At the same time, patients involved in observation had demonstrated poor prognosis regarding malnutrition screening using the MUST score, which revealed an increase in the number of moderate to high-risk patients over the observation period. In this study, feeding was delivered through both continuous and intermittent methods. Although the continuous feeding method is more recommended for acute brain attack patients over the intermittent feeding method due to its ability to maintain nitrogen balance, both were viewed to cause inadequate compensation for the higher metabolic state and protein reduction (Chapple et al, 2016; Mazaherpur et al, 2016). Therefore, it is recommended that teams adopt a variety of nutritional strategies to enhance effective recovery of brain injury or attacks (Huijben et al, 2018).
Regarding the incidence of feeding-related complications, bedside observation showed that feeding intolerance was the most prevalent complication detected among the observed patients. This feature is congruent with the existing evidence associating the risk of feeding intolerance in 63% of critically ill patients with acute head injury and brain attack as a central neurological response that alters the digestive process and gastrointestinal functioning following brain attack (Chapple et al, 2015; Yuan et al, 2019). Further complications, including aspiration, pneumonia, and diarrhoea were prominent during the observation period. The number of incidences of the previous complications were particularly noticeable between the third and fifth day of observation. Previous research estimated the risk of these complications in relation to the severity of injury evidenced by APACHE score or SOFA. (Sequential Organ Failure Assessment) score, in which poor prognostic findings were associated with energy deficit and higher susceptibility of feeding-related complications (Btaiche et al, 2010; Wang et al, 2013; Yuan et al, 2019).
Recognising the importance of caloric attainment in patients who experience a significant need for energy, the results indicated drawbacks in nutritional outcome. In particular, energy deficit (insufficient caloric intake) was the core issue. The presence of coexisting factors linked to energy deficit accelerated problems such as uncontrolled GRV, which is also influenced by the use of mechanical ventilation (DeLegge, 2011; Zhu et al, 2021). Understanding this relationship would indicate that nursing professionals need to apply an accurate assessment protocol for managing GRV, especially in mechanically ventilated patients (Kalaldeh, 2017). Although using prokinetic agents is a well-established, evidence-based practice to control higher gastric residues and promote tolerance of EN, this study, similarly to a previous study, demonstrated inconsistent usage of prokinetics to handle higher GRV in brain attack patients (Ridley et al, 2011; Zhu et al, 2021).
In fact, the absence of robust nutritional guidelines and protocols in the ICU generates further complexity in assessing and managing patients' complications (Kalaldeh et al, 2015; Dixit et al, 2021). Taking into account the estimated caloric deficit and feeding-related complications, there is potential for rapid deterioration of brain attack patients unless protocols include criteria for regular nutritional assessment (Chapple et al, 2015; Crossfield et al, 2021).
Limitation
Larger sample size would have strengthened the observation outcome. In addiont, observation that extends from a patient's admission to discharge would provide further information about the length of hospital stay and mortality rate. Although the inclusion criteria were specific, classifying patients according to their medical therapy (medical vs surgical, and elective vs emergency) can eliminate the influence of the therapeutic intervention of EN outcomes. Finally, nutritional failure is determined by estimating caloric deficit without protein deficit, which may provide additional justification for alterations in body weight and albumin level in the blood.
Conclusion
Although EN is the most effective nutritional strategy for acute brain attack patients in the ICU, its implementation continues to lack adherence to specialised evidence-based protocols. The most common features of nutritional failure encompassed weight reduction, low serum albumin, feeding intolerance and other feeding-related complications. The vast majority of nutritional failure manifested between the third and fifth day of EN initiation. Factors that were associated with increased probability of EN failure were older age, uncontrolled GRV, length of mechanical ventilation, and some feeding-related complications.
KEY POINTS
- Enterally fed patients with severe head injury or acute stroke were found to be at high risk for nutritional failure
- Signs of nutritional failure included weight loss, high gastric residues, low serum albumin, and inability to attain caloric requirement
- Feeding intolerance was the most prominent feeding-related complications followed by diarrhoea, pulmonary aspiration, pneumonia, and hyperglycaemia
- The highest probability for developing nutritional failure was found to be between the third and fifth day from initiation of enteral nutrition
- Lack of specialised evidence-based protocols for the care of acute brain attack patients added further disparities in nursing practice
CPD reflective questions
- How do nutritional requirements for brain attack patients differ from those of other critically ill patients? Why is this?
- What are the most common observable features of nutritional failure in enterally fed, mechanically ventilated patients who have suffered from severe brain attack?
- When should the intensive care nurse anticipate the highest likelihood of nutritional failure and feeding-related complications?


