Healthcare-associated infections (HCAIs) are a cause of preventable harm that presents both a clinical and an economic burden in the UK and around the world. In the UK, it has been estimated that more than 80% of HCAIs are not present on hospital admission and, therefore, occur during a patient’s stay in hospital (European Centre for Disease Prevention and Control, 2013). In the US, up to 90% of patients admitted to hospital have an intravenous (IV) catheter inserted (Helm et al, 2015), often via a peripheral or central vein (NHS Clinical Evaluation Team, 2018). Patient care often requires insertion of an IV device, whether to administer fluids, parenteral nutrition or blood products or to monitor progress. However, vascular access via these routes is one of the main causes of healthcare-associated bloodstream infections (HCAIs). According to what the author believes to be the most recently available data, about 40% of bloodstream infections in Europe are attributable to the use of IV catheters (European Centre for Disease Prevention and Control, 2013), and it can only be imagined how this may have risen since, given the development of the COVID-19 pandemic over the past few years.
This article outlines some of the literature surrounding catheter-related bloodstream infections (CRBSIs), delves into their clinical challenges and demonstrates their burden on national economic health. It then explores how to achieve good clinical outcomes using national and international guidance and evidence-based practice for the care and maintenance of vascular access devices (VADs). Existing literature focuses on some of the relevant aspects of this issue separately. This article brings these fragments together to present a clearer picture of the situation and lay out a number of solutions to prevent CRBSIs.
Current burden
In 2016-2017, there were an estimated 834 000 HCAIs in English hospitals alone, which was not only a figure close to treble that previously reported by the National Institute for Health and Care Excellence (NICE) (Guest et al, 2020), but also one that led to 28 500 patient deaths and the use of 7.1 million hospital bed days in the same 2016-2017 period (Guest et al, 2020). According to the most recent data for England, CRBSIs account for about 10-20% of HCAIs in the UK (National Biofilms Innovation Centre, 2022), and up to 70% are considered preventable (Clare and Rowley, 2021).
Specific examples of progress include a 2-year programme to reduce infection rates related to central venous access in intensive care, reported by Bion et al (2013). However, since 2020, while official government data have been lacking, anecdotal evidence indicates that some hospitals have noted a rise in CRBSIs, as resources have been sparse, and the use of IV therapy and vascular access devices (VADs) have played a central role in treating patients with COVID-19 (Barton, 2022).
Morbidity and mortality
CRBSIs are a significant cause of morbidity, mortality and increased length of stay in hospital, particularly in the intensive care unit (ICU) (Olaechea et al, 2013; Ferroni et al, 2014).
An older systematic review of 39 studies published between 1984 and 2012 across 14 geographical regions around the world placed the overall CRBSI rate between 0.38 and 4.58 per 1000 catheter days (Dreesen et al, 2013). More recent data specific to the UK place the CRBSI rate on the lower end of this range, at 0.38 episodes per 1000 catheter days (Bond et al, 2022). In 2009, a review performed on available data of intensive care units in four European countries (UK, France, Italy and Germany) estimated between 8400 and 14400 CRBSI episodes per year (Tacconelli et al, 2009), while, in the US, about 250 000 bloodstream infections are acquired every year, reported to be the third leading cause of hospital-acquired infection (Gahlot et al, 2014).
While researchers widely agree on the impact of CRBSIs on mortality, statements regarding this appear widely unreferenced across the literature, although a recent review in the UK reported 0.01 CRBSI deaths per 1000 catheter days (Bond et al, 2022). Across Europe, mortality ranges from 1000 to 1584 deaths per year (Tacconelli et al, 2009), with attributable mortality in Spain, for example, estimated to be 9.4% (Olaechea et al, 2013). In 2004, Wisplinghoff et al (2004) found that, over a 7-year observation period, the crude mortality rate of blood stream infections was 27%.
Economic burden
HCAIs are estimated to cost the NHS in England about £2.7 billion per year (Clare and Rowley, 2021). For a European example, the cost of CRBSIs in Italy varies greatly (€4080—€14 800 per patient) but averages at €5575 (Mandolfo et al, 2019). In the US, CRBSIs cost more than 2 billion a year, based on an average cost of care of $45 000 per patient (Rupp and Karnatak, 2018).
Sources of contamination
A CRBSI is defined as the presence of bacteraemia originating from an intravenous catheter (Gahlot et al, 2014). Several sources of contamination can lead to a CRBSI. According to epic3 guidelines, the source of most CRBSIs are the microorganisms that colonise catheter hubs, as well as the skin adjacent to the insertion site (Loveday et al, 2014).
Central venous catheters (CVCs)-also referred to as central venous access devices (CVADs)-in particular pose a greater risk infection than any other medical device, and they are known as the main source of bacteraemia and septicaemia in hospitalised patients (Gahlot et al, 2014). For example, the relative risk for CRBSI is up to 64 times greater with CVCs than with peripheral venous catheters (PVCs) (Gahlot et al, 2014). The most likely primary source of CVC-related infection depends on how long the device is in situ: in short-term use (less than 10 days) this is colonisation by cutaneous organisms along the external surface of the catheter, while in long-term use (more than 10 days) it is intraluminal spread from the hub (Gahlot et al, 2014).
The tip of the catheter and cutaneous tract may be colonised with skin flora, or the lumen may be colonised because of being extrinsically contaminated prior to insertion. Less commonly, the internal surface of the lumen may also be contaminated either because of contaminated infusate or medication or because of haematogenous seeding from another more distant infected site (Gahlot et al, 2014). Common endogenous infectious agents include the patient not washing their hands following toileting or touching their skin and then touching the IV site or hub (Lavery, 2010). Exogenous infectious agents that are relatively common include transfer from the hands of a healthcare practitioner, highlighting the importance of hand hygiene (Box 1), or contact with other patients (Lavery, 2010). However, according to Gahlot et al (2014), 60% of CRBSIs were caused by microorganisms from the patient’s skin itself.
Box 1.Best practice for hand hygiene before and after contact with catheter or insertion site
- Wash hands with non-antimicrobial liquid soap and water when:
- Hands are visibly soiled or dirty
- Potentially contaminated with blood or body fluids
- Caring for patients with vomiting or diarrhoeal illnesses
- Caring for a patient with a suspected or known gastrointestinal infection, e.g. norovirus or a spore-forming organism (such as Clostridioides difficile)
- In all other cases and for routine hand hygiene during care, use alcohol-based hand rubs (which should be available for staff as near to point of care as possible)
Source: Loveday et al (2014), NHS England (2022)
Common microorganisms that reside on the skin (and may be transferred from either the patient or the health professional’s hands) include Staphylococcus epidermidis and Staphylococcus aureus (Lavery, 2010). Enterococcus spp, Pseudomonas spp, Serratia spp and Enterobactor spp may also originate in the bowel flora and be transferred via the patient’s hands or equipment (Lavery, 2010). However, according to epic3 guidelines, coagulase-negative staphylococci, mainly S. epidermidis, are the most common causes of CRBSI, followed by other microorganisms such as S. aureus, Candida spp and enterococci (Loveday et al, 2014).
In addition, biofilm forms a slimy coating around the lumen, to which bacteria can then easily adhere, entering the bloodstream from the point of the VAD insertion through the skin or contaminated parts of the catheter (Caguioa et al, 2012). Resistance to antibiotic therapy due to biofilm formation also has an important role in development of bacteraemia (Gahlot et al, 2014).
Best practice strategies
Hand hygiene
Hand hygiene has been confirmed to be an important factor in infection prevention (World Health Organization (WHO), 2009), while also being the easiest. Best practice for hand hygiene is summarised in Box 1. Once hands are decontaminated, clean and non-sterile gloves must be worn before coming into contact with the catheter or closed system (Loveday et al, 2014).
Aseptic Non-Touch Technique
The Aseptic Non-Touch Technique (ANTT) clinical practice framework (Figure 1) was developed in response to a lack of standardisation of a safe aseptic technique and a common language for education, research and clinical practice, resulting in variability in practice standards and concerns for patient safety (Rowley and Clare, 2020; ANTT, 2021).
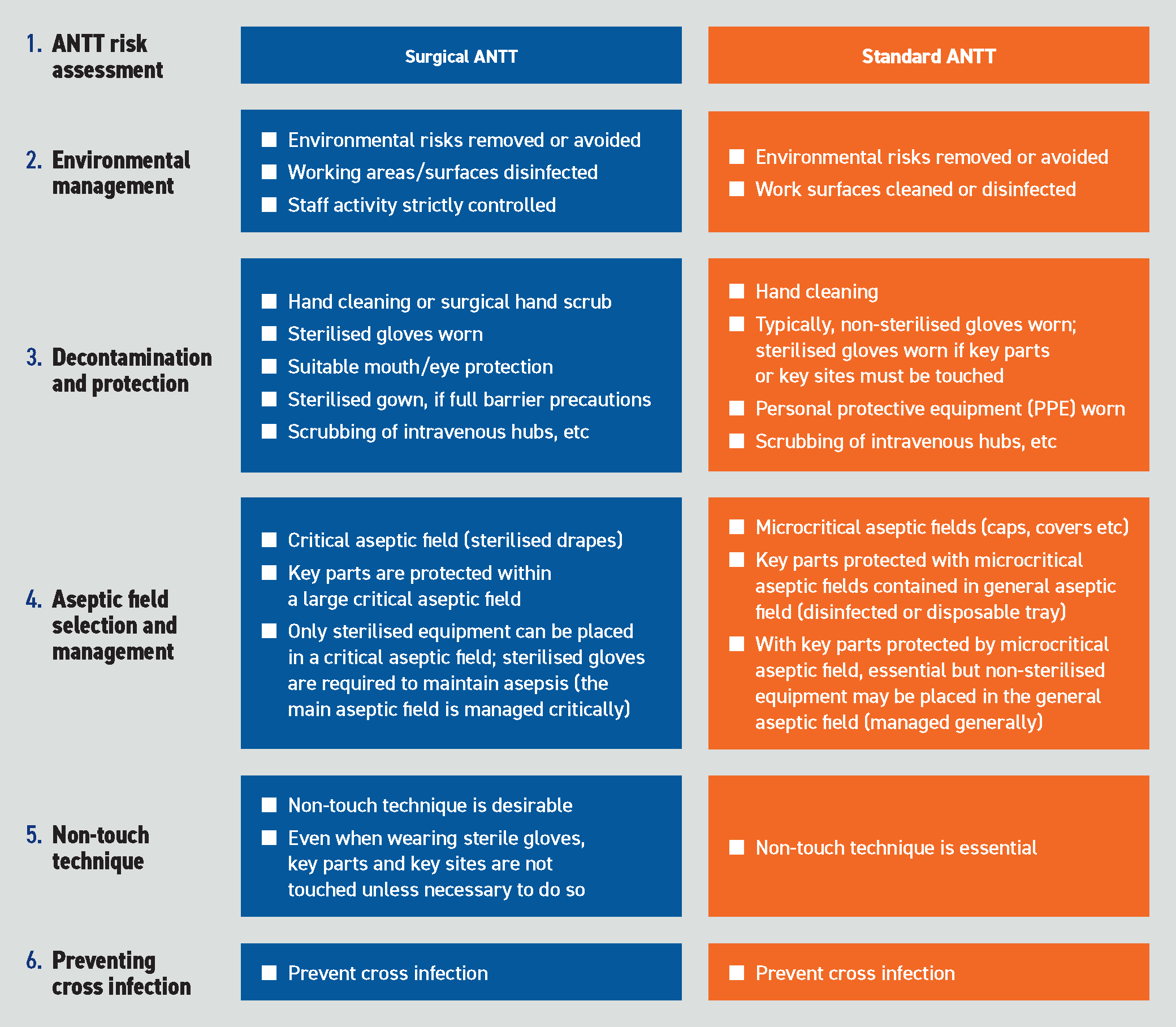
ANTT is recognised as best practice for vascular access by NICE (2012), the Association for Vascular Access (Rowley and Clare, 2019) and the Infusion Nursing Society (Gorski et al, 2021). It is one of the most commonly performed infection prevention strategies, used by 88% of NHS Trusts in England as their single standard aseptic technique (Rowley and Clare, 2020).
ANTT is intended for use with any invasive procedure that involves risk of infection to the patient. The aim is to always maintain asepsis, and this is achieved through a concept termed key-part and key-site protection. This involves the integration of standard precautions, sterile supplies, non-touch technique and aseptic fields, as well as following the principal practice rule that key parts must only come into contact with other key parts and key sites (ANTT, 2021).
Skin antisepsis of insertion site
Importantly, disinfection of the skin prior to invasive medical procedures can serve as an effective measure for the prevention of microbial contamination. In fact, as the risk of infection is known to increase with the density of the microorganisms around the insertion site, antisepsis (decontamination/preparation) of the insertion site is among the most important measures in preventing CRBSI (Loveday et al, 2014; Gorski et al, 2021).
The epic3 guidelines recommend precautions during catheter insertion (Loveday et al, 2014). The access site should be decontaminated with 2% chlorhexidine gluconate in 70% alcohol (or povidone iondine in cases of sensitivity) applied with friction and allowed to air dry (Loveday et al, 2014).
A cross-hatching back-and-forth technique has been shown to be 10-times more effective at reducing bacterial load than a circlular approach (McDonald et al, 2001). It enables maximum contact between the skin and antiseptic preparation, encouraging the solution to make its way to the skin’s deeper cell layers (Silva, 2014).
Maintenance of vascular access devices
Appropriate care and maintenance of VADs are essential for preventing CRBSIs, because components such as the hub and lumen can harbour sources of infection. Infection risk can be minimised through use of ANTT (Lee and Terry, 2021). Other important principles of care include maintaining a closed IV system with minimal connections, maintaining the patency and correct positioning of the catheter, and preventing damage to the device and associated equipment (Lee and Terry, 2021).
When bacteria manage to adhere to the surface of the catheter, this can facilitate the formation and growth of attached bacterial communities called biofilms (National Biofilms Innovation Centre, 2022). Bacterial biofilms lead to survival advantages, such as potential virulence, pathogenesis of infection and resistance to antibiotics; the persistence of staphylococcal infections is an example of this (Hoiby et al, 2011; Brandwein et al, 2016). The flushing of IV catheters following a push-pause (pulsatile) flushing technique is an effective method in reducing catheter bacterial colonisation to prevent CRBSIs (Ferroni et al, 2014).
The skin’s microbiome also plays a role in bacterial infection and impacts the way a biofilm community behaves (Percival et al, 2012). For example, moist skin harbours different bacterial species than does dry skin; therefore, a specific bacteria’s ability to exploit the skin’s barrier is partially dependent on its microbiota (Brandwein et al, 2016). Cleaning the exit site with 2% chlorhexidine in 70% alcohol and allowing to air dry on a regular basis and the use of a sterile, transparent semi-permeable polyurethane dressing to cover the intravascular insertion site (changed every 7 days or sooner if needed with the use of ANTT and skin asepsis) can help minimising CRBSI originated at insertion site, as they provide a barrier to external contaminants (while still allowing moisture vapour and skin breathability) (Loveday et al, 2014).
Care bundles approach
The care bundle approach consists of implementing a group of individual evidence-based best practice interventions for a variety of clinical purposes, including infection prevention. Care bundles for intravascular devices would aim to reduce the number of IV devices in situ and prevent infections in those devices that are needed. However, implementation of care bundles requires careful multidisciplinary team planning and consensus.
As the name suggests, care ‘bundles’ should never be broken up and used in an ad-hoc way. They are created intentionally to be used together to improve patient outcomes using a standardised approach based on robust evidence and guidelines, such as epic3 and INS 2021 (Loveday et al, 2014; Gorski et al, 2021). The use of care bundles helps healthcare professionals to standardise best evidenced practice while reducing variability or guesswork in the provision of care (Clare and Rowley, 2021).
An example of a successful care bundle for vascular access was the HANDS quality improvement project carried out at King’s College Hospital NHS Foundation Trust (Caguioa et al, 2012). The HANDS mnemonic used as part of this Trust-wide initiative stands is expanded in Box 2.
Box 2.HANDSH Hand hygieneA Antisepsis (using 2% chlorhexidine gluconate in 70% isopropyl alcohol)N Non-touch techniqueD Date on a clear IV film dressing, daily inspections and documentationS Scrubbing the hub for 15 seconds, allowing it to dry
Another initiative that has been successfully applied to IV management is the Department of Health and Social Care’s high-impact interventions from the Saving Lives programme, which was launched to help healthcare organisations ensure that robust infection-prevention measures are embedded across their acute trusts (Aziz, 2009; Coghill, 2009). In particular, highimpact intervention number 1 is the central venous catheter bundle (DHSC, 2007a). High-impact intervention number 2 is the peripheral IV cannula care bundle (DHSC, 2007b), which has been shown to improve peripheral IV cannula care, resulting in reduced rates of meticillin-resistant Staphylococcus aureus (MRSA) bacteraemia rates (Aziz, 2009). The actions outlined in these care bundles are concerned with insertion and ongoing care. Insertion actions cover aspects such as catheter type and insertion site, hand hygiene, personal protective equipment, skin preparation, dressing choice, safe disposal of sharps and appropriate documentation (DHSC, 2007a; 2007b). Ongoing care actions include appropriate hand hygiene procedure before and after every patient contact; ensuring intravenous devices are still clinically indicated; at least daily inspection of the catheter site for signs of infection; use of an intact, dry, adherent transparent dressing; use of aseptic technique during catheter access; administration set replacement at appropriate intervals (i.e., blood products immediately, parenteral nutrition after 24 hours, others after 72 hours); and appropriate rather than routine catheter replacement (DHSC, 2007a; 2007b). Each action in the bundle is important, but infection reduction occurs when all actions are performed every time for every patient.
Reducing vascular access device infection by best-evidenced practices
Some additional best practices for reducing VAD infection, facilitating catheter maintenance and, therefore, improving clinical outcomes include the following:
- Using a VAD with the minimum number of ports or lumens needed for the care of the patient (Loveday et al, 2014)
- Using a designated single-lumen catheter to administer lipid-based solutions (Loveday et al, 2014)
- Consideration of a chlorhexidine-impregnated sponge dressing (Loveday et al, 2014)
- Consideration of daily cleansing with chlorhexidine in adult patients with a CVC (Loveday et al, 2014)
- Correct positioning and securement of IV cannulas to reduce risk of mechanical phlebitis or infection (Higginson, 2015).
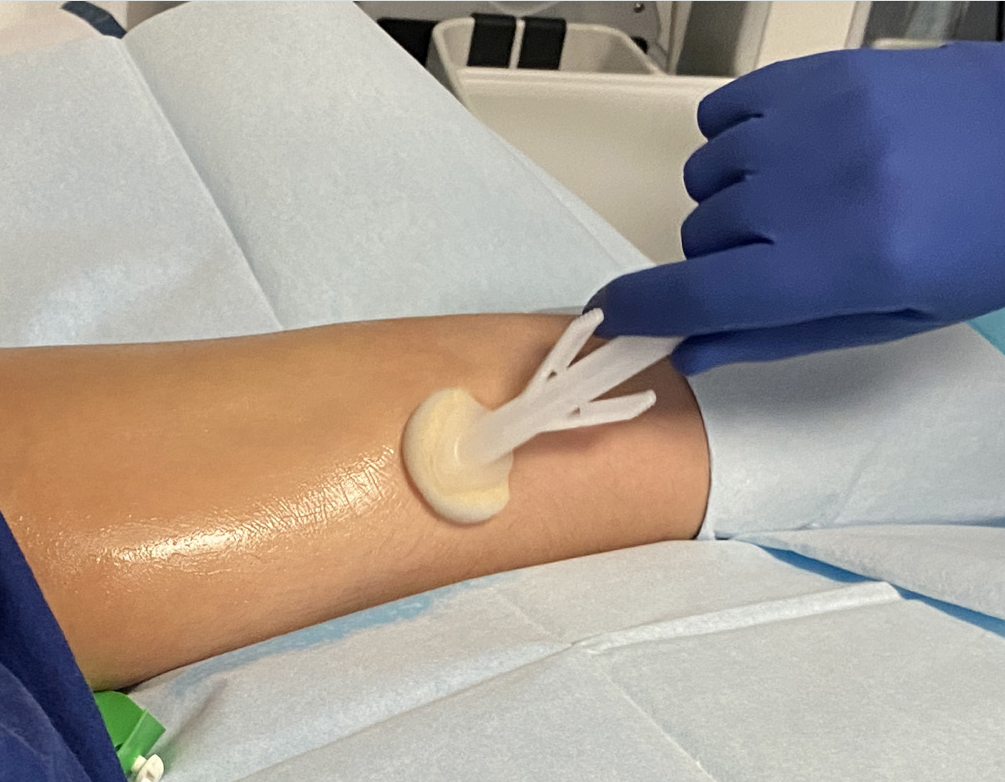
Skin antisepsis with a single-use applicator or wipes
The epic3 guidelines advise decontamination of the skin at the insertion site with a single-use application of 2% chlorhexidine gluconate in 70% isopropyl alcohol (or povidone iodine in alcohol for patients with sensitivity to chlorhexidine) (Loveday et al, 2014). While fibre-based wipes impregnated with chlorhexidine/isopropyl alcohol solution can be used with effective non-touch technique, they bring the health professional’s fingers closer to a key site and introduce more vulnerability to human factors. They also increase variability in terms of the volume of solution each wipe contains, the way that they are held (e.g., folded, open or scrunched up) and interpretations of method of use (Clare and Rowley, 2021). To date, BD ChloraPrep™ single-use applicators (Figure 2) are the only applicator-shaped product licensed to use for this purpose by the Medicines and Healthcare products Regulatory Agency (MHRA) (Clare and Rowley, 2021).
Flushing and locking
Best-evidenced practice advocates flushing of the device after any IV drug administration (to prevent any mixing of incompatible medicines or solutions) and at regular intervals to promote and maintain patency (Ferroni et al, 2014). An IV flush is administered normally with 0.9% sodium chloride. The correct techniques of pulsatile flush and positive pressure disconnection should be used (Royal College of Nursing (RCN), 2016; Gorski et al, 2021). Peripheral IV cannulas must be removed or effectively flushed at the end of an IV procedure, particularly where anaesthetic or sedative drugs have been administered and between each new drug (NHS England, 2017). Otherwise, residue of these drugs can later be introduced to the patient’s circulation and cause muscle paralysis, unconsciousness and respiratory and cardiac arrest (NHS England, 2017).
Flushing and locking are strongly associated with intraluminal occlusion following build-up of fibrin and/or infusion fluid deposits or a mixture of incompatible medications and solutions (Goossens, 2015). Regarding the appropriate lock solution for occlusion prevention, the Italian group for VADs (GAVeCeLT) highlights that this is based on proper flushing technique and locking with saline. For infection prevention, lock solution should include substances with antibacterial and antibiofilm activity, such as citrate and/or taurolidine, and, furthermore, evidence does not support the use of the heparin lock in non-dialysis catheters (Pittiruti et al, 2016).
Appropriate flushing with the correct solution and technique also helps to remove potential nesting material for microorganisms and can reduce the risk of chemical phlebitis (Ferroni et al, 2014). This is pertinent, because phlebitis has been cited to be the most common IV complication, occurring in up to 96% of patients, according to data from 1985 (Niël-Weise et al, 2010).
However, Hadaway (2006) made the important point that correct technique is only a part of the puzzle, with the technology of catheter flushing also playing an essential role. The flush solution, along with the source of the solution and the design of the syringe, mechanical pumps, needle-free injection systems and catheter, combine with appropriate technique to achieve effective catheter flushing (Hadaway, 2006). The use of syringes with at least a 10 ml are recommended for long-term CVCs, as well as in cases where catheters and ports are not designed to withstand the high pressure of power injection (Goossens, 2015). Guidelines recommend a flushing volume of at least twice that of the catheter and add-on devices (i.e., 5-10 ml, depending on VAD type), but a flush volume of 20 ml is recommended after an infusion of more viscous products or after blood sampling from the device (Goossens, 2015).
Pre-filled syringes
Pre-filled 0.9% sodium chloride syringes may provide a costeffective, time-efficient and more standardised alternative to manually drawing up a syringe for flushing, as demonstrated in an assessment of paediatric intensive care (Ceylan et al, 2021). They produce less waste and reduce the risk of needlestick injuries and contamination, as well as the risks of labelling confusion and potential errors, which may lead to patient harm. Using separate products to prepare an IV flush increases the number of steps, products and sometimes people involved and, thus, increases the risk of human error (Lee and Terry, 2021). Pre-filled syringes are registered as medical devices. However, the use of a saline ampule to flush a device is categorised as prescription only medicine. Therefore, moving from manually flushing to using a pre-filled syringe simplifies the overall process by negating the need for a prescription.
Lee and Terry (2021) described how, when manually drawing up an IV flush, each ampoule of sodium chloride 0.9% is classified as a prescription only medication (PoM) and requires a separate prescription, patient-specific direction or patient group directive. A pre-filled syringe is not defined or classified as a medicine but as a CE-marked medical device. Medical devices are classified into four classes according to increasing levels of risk (MHRA, 2021a). In the UK, pre-filled syringes for the purpose of flushing a medical device, such as a catheter or a port, are class II or class III (MHRA, 2021b). Unlike other pre-filled saline syringes that are classified as class IIa CE devices, BD PosiFlush™ Pre-Filled Syringes are classified as class III medical devices, meeting more-stringent mandatory requirements (Official Journal of the European Union, 2017).
Conclusion
The placing of IV catheters is integral to patient care. However, it is not without risks, and every precaution should be taken to reduce and prevent the occurrence of CRBSIs. Hand hygiene, aseptic technique and full-barrier precautions (where and when necessary) should always be used. The skin should be properly disinfected prior to insertion, and the catheter should be appropriately secured, monitored and maintained following evidence-based practice guidelines. Unnecessary catheters should be removed (or not placed to begin with). Quality improvement interventions can support the management of CVCs and PVCs to ensure correct maintenance and timely removal (Loveday et al, 2014). Regular education and assessment of health professionals in their competence and regular adherence to CRBSI prevention best-evidenced practices are also paramount to achieving reduced infection and good patient outcomes.

