Negative pressure wound therapy (NPWT) is an effective, advanced wound care approach for treating and managing multiple acute and chronic wound aetiologies, including, for example, surgical incisions, diabetic foot ulcers and pressure ulcers/injuries. In all currently applied NPWT methods the wound is covered by an airtight dressing, and negative pressure is then applied to remove excess exudate and mechanically stimulate the wound environment, to initiate and progress the tissue repair process. This concept was first introduced in the form of stationary NPWT systems designed for use in a hospital setting (which are still in use), but which later evolved into portable, single-use (disposable) NPWT systems for treating individual patients in their own environment.
Regardless of whether an NPWT system is stationary or portable, tissue deformations – occurring at the macroscopic and microscopic scales due to the applied negative pressure – are consistently listed in the literature as a fundamental mode of action of this wound treatment method (Borgquist et al, 2010; Orgill and Bayer, 2013; Normandin et al, 2021). Saxena and colleagues (2004) were the first to propose that the micro-deformations induced by NPWT systems may promote cell activity towards tissue repair. They based their concept on earlier mechanobiological observations indicating that, in the presence of soluble mitogens (stimulants of immune cells), cells stretched by NPWT tend to become activated, whereas undeformed cells remain quiescent. However, a quantitative formulation of this effect has only recently been reported (Orlov and Gefen, 2022a).
As Orlov and Gefen (2022a; 2022b) have described, during the proliferation phase of wound healing, dermal fibroblasts and myofibroblasts proliferate and migrate from the periwound skin into the wound bed, in order to synthesise growth factors; these cells then produce and contract the collagen bundles that eventually form scar tissue (Li and Wang, 2011; Darby et al, 2014; Jiang and Rinkevich, 2020). Accordingly, (myo) fibroblast migration from the periwound skin into the wound bed is essential for healing (Clark et al, 2003). Myofibroblasts are typically activated fibroblasts that apply contractile forces on the extracellular environment and can collectively generate tissue tension. The latter occurs as part of the normal scarring process for skin closure, but can also form pathologically in excess, resulting in skin contractures (Tomasek et al, 2002; Peng et al, 2021).
Given that fibroblasts and myofibroblasts reside predominantly in the periwound skin, the periwound is considered to be the biological reservoir of these cells for wound healing and tissue repair. Clinically, the periwound area has been defined as ‘the defensive zone that contains the wound’ (Dowsett et al, 2015), and is often described as the skin region within about 4 cm from a wound's edges (Colwell et al, 2011; Thayer et al, 2016). However, a recent clinical consensus document noted that it is difficult to quantify this area for specific wounds according to distance per se, because the size of the area in the case of an individual patient and specific wound may be related to underlying wound pathology, dressing, device, treatment method, or skin conditions and microbiome status (LeBlanc et al, 2021). In addition to being the habitat for tissue-repairing cells, the periwound region also contains the vascular supply to the wound which, likewise, is critical for normal healing. Indeed, periwound hypoxia is known to delay wound healing (Woo et al, 2015), whereas pharmacological stimulation of the vasculature to promote angiogenesis (eg through targeted delivery of specific growth factors) helps increase the vascular supply to the wound bed, supporting the healing process (Grundmann et al, 2007; Ma et al, 2016; Veith et al, 2019; Kong et al, 2022).
Periwound tissue deformations induced by NPWT mechanically stimulate (myo)fibroblasts to proliferate and migrate from intact skin into the wound bed, where their biological activities are necessary for wound healing (Saxena et al, 2004; Aljghami et al, 2019). These activities include cytokine signalling, production of basic fibroblast growth factor (which also promotes angiogenesis), and expression of collagen type I and matrix protein secretion; this is followed by increased collagen fibrillogenesis to generate traction and contractile forces, to promote wound closure and support newly formed scar tissue (Saxena et al, 2004; Wilkes et al, 2009; Borgquist et al, 2010; Wiegand and White, 2013; Huang et al, 2014; Wiegand and White, 2014; Katzengold et al, 2021). That is, the stimulation of (myo)fibroblasts to proliferate and migrate from the periwound skin, and later produce collagen in the wound bed, by inducing suitable mechanical deformations at the cell niche (deformations that are delivered into the cell bodies for cell activation), is essential for promoting wound healing in general; it is also critical for triggering the repair of chronic wounds in particular.
Nevertheless, the level of mechanical stimulation delivered to the periwound area by an NPWT system must be optimal, as per the well-known ‘Goldilocks principle’ in biology. Namely, that if the tissue deformations are too low, they will not be sensed by the target cells, or by a sufficient number of these cells, the result being that they may not have a positive effect on the wound healing process. Alternatively, if deformations in the periwound area are excessively high, they may cause damage to cells or tissues, leading to wound deterioration. At the cell scale, the mechanical deformations need to be at a sufficient level to be detected by the mechanoreceptors on the plasma membrane of the target cells, eg by primary cilia (Christensen et al, 2008; Hosio et al, 2020).
When these mechanical stimuli are detected by the (myo)fibroblast cells, collective migration towards the wound bed commences, in turn facilitating collagen deposition (Wiegand and White, 2013). Another positive consequence of a successful mechanical stimulation by an NPWT system is angiogenesis and maturation of the microvasculature within and around the wound bed, to support the formation of granulation tissue on the basis of the neovascular supply (Erba et al, 2011; Huang et al, 2014; Ma et al, 2016).
The optimal level of cell and tissue deformations for gaining the biological benefits of NPWT without risking cell and tissue damage – according to the ‘not too little, nor too much’ Goldilocks principle – was investigated by the research group led by the author and reported in multiple articles (Slomka and Gefen, 2012; Toume et al, 2017; Katzengold et al, 2021). Specifically, we found that exposing fibroblast cultures to static mechanical strain (dimensionless deformation), at a level of 3%, increased their migration velocity and reduced gap closure times relative to unstretched control cell cultures (Toume et al, 2017; Katzengold et al, 2021). However, exposure to 9% strain or higher levels led to plasma membrane poration, followed by apoptotic cell death (Slomka and Gefen, 2012). (Mechanical strain is a geometric, dimensionless measure of the extent of deformation, representing the relative displacement between two points within or on the surface of a material.)
Importantly, applying contemporary bioengineering computer modelling enables the optimal range of positively stimulating deformations to be quantified for each existing or new NPWT system. This supports the assessment of how effectively NPWT will stimulate the periwound, to activate and accelerate (myo)fibroblast proliferation and migration (Orlov and Gefen, 2022a).
How the influence zone is quantified in bioengineering work
The published work of the author and his research group, to determine the strain sensitivity threshold that triggers or promotes the collective migration of fibroblasts, revealed that 0.5% strains are a suboptimal mechanostimulus. That is, although this strain level does not damage the cells, neither does it have a positive effect on promoting cell migration (Katzengold et al, 2021). Subjecting fibroblast cultures to a 3% strain level increased the rate at which they migrated to close a gap formed by an in vitro injury, but the above effect was reduced under 6% strain (Toume et al, 2017). Other evidence reviewed in our aforementioned published works (Katzengold et al, 2021) consistently indicated that strains above 5% slow down cell migration.
A conservative assumption based on the above published research (Slomka and Gefen, 2012; Toume et al, 2017; Katzengold et al, 2021; Orlov and Gefen, 2022a) is that the optimal range of mechanical strains for stimulating collective fibroblast migratory behaviour without compromising (other) cell and tissue function is between 0.5% and 3%. For practical purposes, and particularly for the purpose of computational (finite element) modelling of the biomechanical effects of NPWT systems on the wound bed and periwound tissues, a discrete strain-stimulation threshold value is required. In our recently published work (Orlov and Gefen, 2022a), for simplicity, we considered this threshold as the mid-range of the above optimal value, ie 1.75% strain. This implies that periwound skin strain equal to, or exceeding, the 1.75% strain threshold, is associated with a high likelihood of inducing a fibroblast migratory response towards the wound bed. In contrast, skin regions subjected to strains of below 1.75% are considered less likely to exhibit such a positive migratory response.
Importantly, as the skin strain field decays with distance from the site of applied negative pressure (which is directly above the wound bed), there must exist an influence zone around the wound specific for the NPWT system type used to treat a given wound. Furthermore, it is essential for this influence zone to be of adequate magnitude and area to result in a positive clinical outcome; that is, the influence zone must reach sufficiently far into the periwound region to promote healing and also have a biologically effective strain intensity within this domain.
The influence zone of an NPWT system is therefore defined as the effective periwound region size from which it is possible to recruit dermal (myo)fibroblasts to promote their migration into the wound bed via mechanical stimulation (Figure 1). The influence zone is well established in the field of orthopaedic mechanobiology, where the term has been used to describe the mechano-responsiveness of bone cells (osteocytes) as a function of their distance from the origin of the mechano-stimulation (Nicolella et al, 2005; Baïotto and Zidi, 2009).
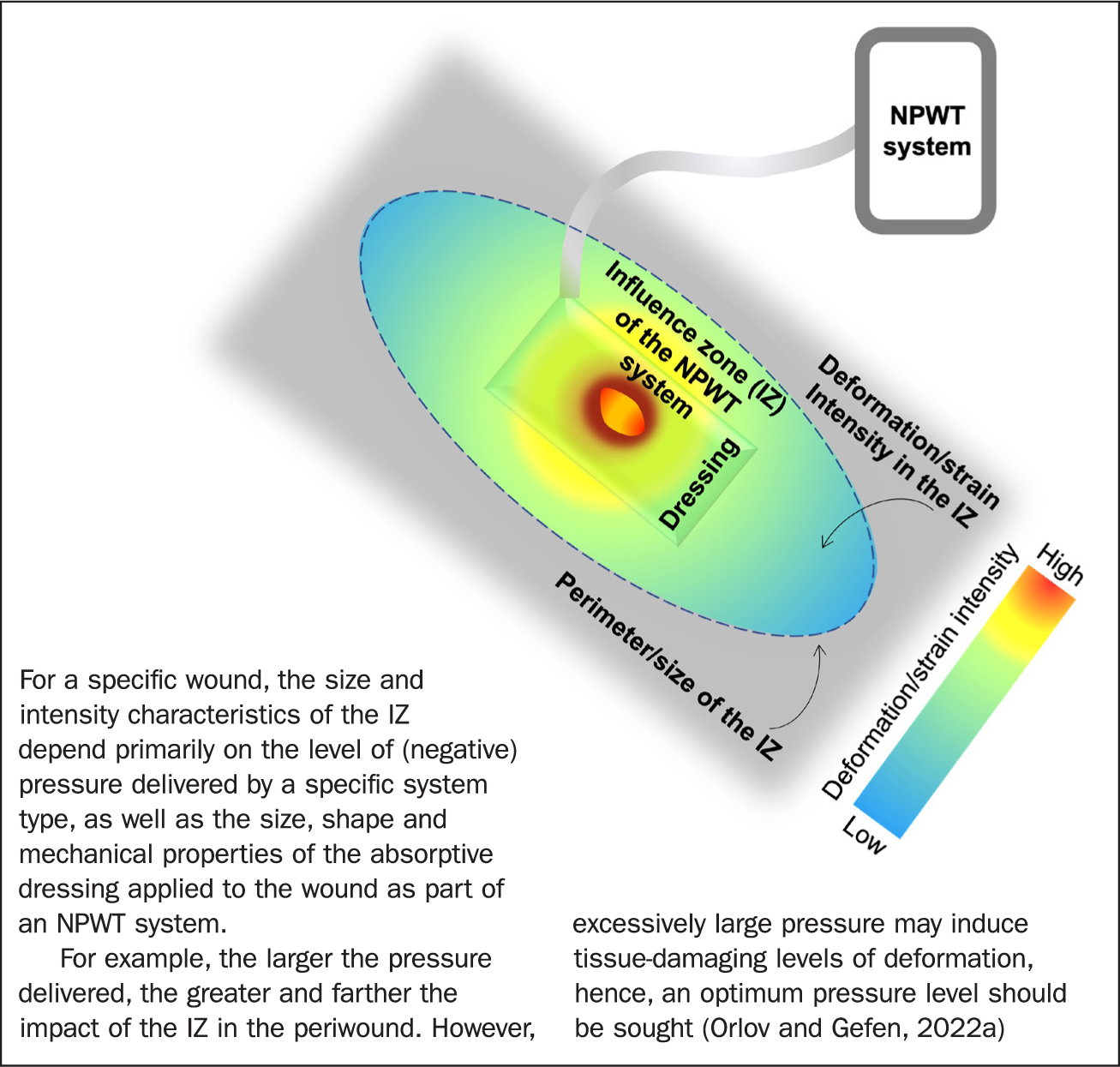
There is supporting evidence from clinical and laboratory studies of the importance of the magnitude of applied negative pressures for positive biological influence on the wound-healing process, for example in treating diabetic foot ulcers (Al-Sabbagh et al, 2020), infected wounds in a pig wound-healing model (Zhou et al, 2013), and non-infected wounds in a rabbit model (Chen et al, 2019). These studies consistently indicated that a potential decrease (loss) of absolute negative pressure delivered to the wound de facto, which results in a reduction in size and intensity of the influence zone, is likely slow or halt the healing process.
In NPWT systems that use traditional foam and film dressings the application of negative pressure is concentrated at the wound bed only. However, in NPWT systems designed to use absorptive dressings, there is potential for the negative pressure to be distributed also over the periwound area. In other words, an absorptive dressing covers both the wound bed and the periwound, enabling the influence zone to extend over a larger area and be of greater magnitude. This potentially leads to greater stimulation of available and responsive biological resources (in the periwound tissues) by including a larger reservoir of (myo)fibroblasts that can potentially be recruited and made available for wound healing (Figure 1) (Orlov and Gefen, 2022a).
Orlov and Gefen (2022a) computationally analysed two distinct design concepts for single-use NPWT systems operating with absorptive dressings: canister-based versus canisterless. Canister-based technology provides intrinsic stable delivery of the intended negative pressure level, because exudate fluids are constantly transferred from the wound into a canister, preventing a state of dressing saturation. In contrast, in a canisterless system, where the delivery of negative pressure depends on continuous and sufficient evaporation of wound fluids from the dressing, there may be a loss of the intended wound-bed pressure due to dressing saturation.
To investigate whether these two single-use NPWT systems differ in their influence zone characteristics, Orlov and Gefen (2022a) used advanced finite element modelling, representing an open wound covered by an NPWT dressing. The modelling showed that, in the canisterless system, a reduction in negative pressure below 40 mmHg at the wound bed adversely lowered periwound tissue mechano-stimulation around a 120 mm × 70 mm size wound to less than one-third of baseline mechano-stimulation level. Further pressure reduction to 20 mmHg or lower resulted in a complete lack of periwound stimulation in the canisterless system (Orlov and Gefen, 2022a). Although the above results clearly demonstrated that consistent delivery of the intended pressure level is critical for maintaining an effective influence zone throughout the treatment period, there is a case for further bioengineering research into additional factors, such as how the influence zone of an NPWT system around a given wound is affected by dressing shape and size.
Conclusion
The influence zone provides quantitative bioengineering performance metrics for NPWT systems, indicating the effectiveness of mechano-stimulation of the periwound, namely, how far from the wound bed a specific NPWT system is able to deliver mechano-stimulation into the periwound, and at which intensity within that domain.
The influence zone is therefore an objective and standardised measure of one of the fundamental modes of action of NPWT systems: their ability to effectively and optimally deform the periwound macroscopically and microscopically (complementary to adequate fluid handling). Importantly, this influence zone must be of biologically influential deformation levels and extend sufficiently into the periwound in order to stimulate (myo)fibroblasts to migrate and progress wound healing, without overstretching the tissues in order not to cause or worsen cell and tissue damage.
Incorporating the influence zone theory into the design of research studies to investigate the efficacy of NPWT systems facilitates the quantitative systematic comparison and contrast of existing and potentially new systems. Ultimately, this enables the performance of different systems to be rated, which can be used to guide not only further research and development work, but also clinical decision-making. Research undertaken by the author and his colleague (Orlov and Gefen, 2022a) found that an effective influence zone requires, first and foremost, continuous delivery of the intended pressure to the wound and its surroundings throughout the treatment period.
KEY POINTS
- The influence zone is an innovative, key quantitative performance metric for NPWT systems
- It indicates the effectiveness of mechano-stimulation of the periwound, ie how far from the wound bed a specific NPWT system is able to deliver mechano-stimulation into the periwound, and at which intensity, to attract migration of tissue-repairing cells
- Continuous and consistent delivery of the intended pressure level by an NPWT system is critical for maintaining an effective influence zone throughout the period of wound treatment
CPD reflective questions
- What is the influence zone, and why is it important in efficacy research of NPWT systems?
- How would inconsistency in delivering the intended pressure level, so that pressure reduces over time, affect the influence zone size and its intensity?
- Consider how purchasing decisions and clinical decision-making would be affected if manufacturers reported the influence zone metrics for each existing and new NPWT system and associated dressing types?


