Peripheral intravenous catheter (PIVC) insertion is often the first medical procedure undertaken on hospitalised children to facilitate medical treatment (Ullman et al, 2019). PIVCs are small, hollow, flexible tubes, which are inserted into a peripheral vein over a needle. Typically, PIVCs are inserted into the upper or lower limbs in children using the traditional landmark (palpation and visualisation) insertion technique (Ullman et al, 2020). Clinicians report PIVC insertion in children is often difficult, and frequently two PIVC insertion attempts, lasting 20-30 minutes, are required before successful insertion (Goff et al, 2013; Tuffaha et al, 2014a; Kleidon et al, 2019). Commonly, children with difficult intravenous access (DIVA) require more than four attempts or needlesticks, and in some extreme situations a PIVC might not be inserted at all (Goff et al, 2013; Kleidon et al, 2019; Archer-Jones et al, 2020). In a systematic review Bahl et al (2021) defined the characteristics of DIVA to be determined by number of failed PIVC insertion attempts (more than two failed insertion attempts), risk factors (vein characteristics including visibility and palpability, patient age, overweight or underweight, chronic health conditions and female gender), and self-report of previous DIVA.
Multiple attempts at PIVC insertion depletes patients of usable veins and delays medical treatment, reducing therapy efficacy, prolonging recovery, and extending inpatient bed days (Goff et al, 2013; Seymour et al, 2014). In recent years, ultrasound has become well established as an integral tool for safe and reliable central venous catheter insertion, in comparison with traditional landmark and open cut-down technique (Bodenham et al, 2016; de Souza et al, 2018; Takeshita et al, 2020; Soundappan et al, 2021). Use of ultrasound allows assessment of vein location, depth, valves, and bifurcations, and provides direct visualisation of the needle path throughout the procedure – a considerable advantage over landmark technique, often referred to as ‘blind puncture’ (Bodenham et al, 2016).
A recent systematic review of PIVC insertion success in hospitalised adults (van Loon et al, 2018) demonstrated that the use of ultrasound increased the success rate for PIVC insertion compared with landmark technique (81% versus 70%; odds ratio (OR) 2.49, 95% confidence interval (CI) 1.37-4.52, P=0.003). Further, in patients with DIVA, ultrasound was demonstrated to be particularly effective in supporting first-attempt insertion success (75% vs 49% first-attempt success: OR 3.23, 95% CI 1.35-7.72). In children, however, there is a lack of rigorous evidence to inform the routine use of ultrasound (McBride et al, 2022). In a systematic review (four studies, 811 participants) of PIVC insertion rates in general hospitalised children (excluding those with DIVA) showed no evidence of an effect for first-time insertion success when ultrasound guidance was used compared with landmark technique (RR 1.27; 95% CI 0.90-1.78) (Kleidon et al, 2022). In contrast, in another systematic review (Kleidon et al, 2021) that included all children, including those with DIVA (four studies, 592 patients), PIVC insertion success improved with ultrasound guidance (RR 1.60; 95% CI 1.02–2.50). The effect size increased (RR, 1.87; 95% CI, 1.56–2.24) in high-risk cohorts such as children with DIVA (three studies, 309 patients) (Kleidon et al, 2021; Mitchell et al, 2022).
The clinical value of ultrasound to support PIVC insertion in children with varying degrees of DIVA is yet to be fully appreciated in paediatric patients (Schults et al, 2022). In response to this evidence-practice gap, the authors aim to conduct an open-label randomised controlled trial (RCT) to evaluate the value and effectiveness of ultrasound-guided PIVC insertion in all children with varying degrees of difficult intravenous access.
Aims
The overall aim of this study is to determine the effectiveness of ultrasound-guided PIVC insertion compared with landmark technique to increase first-time insertion success in children aged under 18 years.
Hypothesis
- H0: Children who have their PIVC inserted with ultrasound guidance will have increased first-attempt success compared with landmark PIVC insertion (standard care)
- H1: Children who have their PIVC inserted with ultrasound guidance will have decreased PIVC insertion failure, compared with landmark PIVC insertion (standard care)
- H2: Children who have their PIVC inserted with ultrasound guidance will have prolonged PIVC dwell time compared with landmark PIVC insertion (standard care).
Methods
Study design
The ‘efficacy of PIVC insertion using imaging technology compared to combined palpation and visualisation’ trial, known as the EPIC trial, is a prospective, randomised, parallel-group, single-centre, superiority trial. The protocol is reported in accordance with the SPIRIT 2013 statement (Chan et al, 2013) and subsequent findings will be reported in accordance with trial registration ACTRN 12621000206820 and the Consolidated Standards of Reporting Trials (CONSORT) guidelines (Schultz et al, 2010).
Study setting and sample
The trial is underway. Recruitment began 8 July 2021 and completed in March 2023. The trial is based in a single-site, 349-bed, paediatric tertiary hospital in Brisbane, Queensland. Patients who meet all inclusion and no exclusion criteria (see Table 1) will be eligible to participate in the study.
Table 1. Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
DIVA=difficult intravenous access; PIVC=peripheral intravenous catheter
Intervention
Control arm (landmark technique): PIVC insertion using standard landmark will be performed by a registered nurse or medical officer with pre-existing competence in PIVC insertion using palpation and visualisation of the underlying vessel (as available).
Intervention arm (ultrasound-guided insertion): PIVC insertion by a registered nurse or medical officer with pre-existing competence using ultrasound guidance for PIVC insertion. In addition to standard palpation, ultrasound-guided PIVC insertion uses ultrasound to assess, locate and select an appropriate vein. The PIVC will then be inserted into the vein under direct ultrasound visualisation by advancing the needle into the vein while moving the ultrasound probe in the direction of needle advancement (Takeshita et al, 2019a; 2019b) (Figure 1).
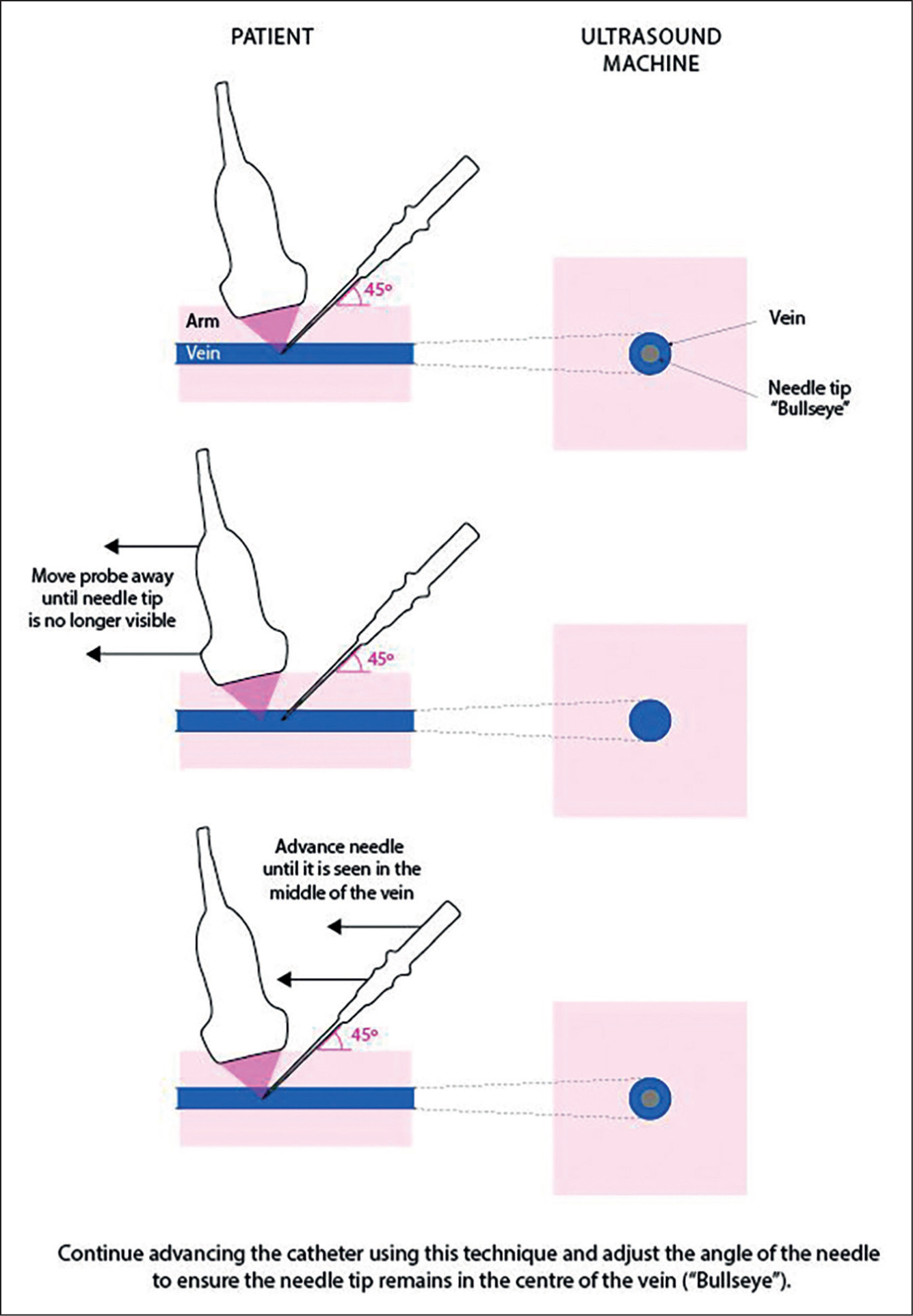
PIVC insertion procedures
The PIVCs will be inserted in a clinical procedure room or patient's bedspace, dependent on patient preference and infection control precautions. Other than technique used for PIVC insertion, all aspects of PIVC insertion, management and removal will be standardised in accordance with Children's Health Queensland (2019a) clinical practice guidelines. Insertion, maintenance and removal will be performed by the usual interdisciplinary clinical staff.
All PIVCs are to be inserted by clinicians trained in PIVC insertion technique (ultrasound guidance and landmark technique), and as per local policy (Vascular Assessment and Management Service, 2019). Aseptic non-touch technique (Children's Health Queensland, 2019b) is to be utilised for all insertions. Catheters will be BD Insyte Autoguard safety PIVC (24G, 22G, 20G etc), BD Nexiva (22G) or B Braun Introcan Safety Deep Access size (24G, 22G); catheter size (gauge and length) will be determined by the inserter, dependent on treatment requirement and vessel assessment. As per international guidelines (Gorski et al, 2021) and local hospital policy (Children's Health Queensland, 2019a), local anaesthetic, both topical and/or subcutaneous, if necessary, will be applied prior to PIVC insertion. Use of sedation prior to PIVC insertion will be at the discretion of the clinician (Children's Health Queensland, 2019a; 2019c). The first insertion attempt will be according to the randomisation schedule, however, in accordance with current international guidelines and local hospital policy, the inserter can choose to change to an alternative insertion technique after one failed attempt (Gorski et al, 2021). The research nurse will collect this data and this will be reported as a secondary outcome for each study group. Devices will be removed, and or replaced by clinical staff when clinically indicated (Rickard et al, 2012; Gorski et al, 2021).
Difficulty of intravenous access will be assessed prior to randomisation using the DIVA Key (Schults et al, 2022), which provides reliable assessment of DIVA status using objective values (patient age and vessel quality) and practice variables (acuity, previous access history). As per the DIVA Key, DIVA status is summarised as low (no clinical urgency, multiple visible and palpable veins, previously well, over 3 years of age and shows minimal anxiety), medium (time critical, few visible/palpable veins, multiple attempts in the past, multiple admissions/co-morbidities, aged under 3 years and shows moderate anxiety), and high risk (urgent insertion, no visible/palpable veins, documented DIVA, severe comorbidities, under 18 months of age, and has severe anxiety/needle phobia).
Outcome measures and definitions
The primary outcome is first-time insertion success, defined as the proportion of PIVCs successfully inserted on the first attempt. Successful insertion is further defined as one skin puncture to achieve successful PIVC insertion, as indicated by the ability to infuse 3-10 ml (appropriate volume determined by patient weight and/or clinical condition) 0.9% NaCl without resistance or evidence of external swelling at PIVC insertion site (Benkhadra et al, 2012; Avelar et al, 2015; Hanada et al, 2017). Secondary outcomes are outlined in Table 2.
Table 2. Secondary outcomes
| Outcome | Definition |
|---|---|
| Number of insertion attempts | Number of skin punctures required to insert device, documented by inserter (or observed by nurse, or other personnel assisting insertion) (Benkhadra et al, 2012) |
| Total PIVC insertion failure | Proportion of PIVCs that are unable to be inserted with the allocated technique and require alternative therapy (Benkhadra et al, 2012 |
| Dwell time | From successful device insertion until PIVC removal (Children's Health Queensland Hospital and Services, 2018) |
| Patient-reported pain on insertion |
|
| PIVC post-insertion failure | Cessation of PIVC function resulting in device no longer being suitable for treatment. Causes of post-insertion failure include:
|
| Consumer satisfaction | Eleven-point numeric rating scale >8 years (Tsze et al, 2018; Birnie et al, 2019)(Measured immediately following insertion and at device removal) |
| Healthcare costs | Associated with insertion procedures, including the cost of subsequent sequalae (eg cost and number of products used, cost of treating complications, staff time for device insertion) (Tuffaha et al, 2014b) |
Sample size
Local and international data report first-attempt PIVC insertion success using landmark technique varies between 35% and 75% (Kleidon et al, 2019; Kleidon et al, 2020; Ullman et al, 2021) Previous work involving the use of ultrasound guidance to insert PIVC demonstrated a 92% first-attempt success rate (Kleidon et al, 2020). Existing RCTs and systematic reviews have reported that ultrasound guidance implementation increases first-attempt success to 80% or more (Stolz et al, 2016; Jauncey-Cooke et al, 2018). The researchers conservatively hypothesise an increase in first-attempt insertion success for children from 60% (landmark technique) to 80% (with ultrasound guidance). With alpha=0.05, 82 children in each group achieves 80% power to detect a difference at least this large, and the results are clinically meaningful, therefore the aim is to recruit 164 children.
Recruitment, randomisation, allocation concealment and blinding
The aim is to identify and approach prospective patients for enrolment via clinical referral to the Vascular Assessment and Management Service (VAMS) and routine screening performed by the VAMS clinical research nurses, Monday to Friday business hours. Patients will be screened for inclusion in the study, approached, and informed consent gained after consultation with the treating team. Research nurses will obtain informed consent and randomise participants using a central web-based service (Griffith University). Randomisation will be in a 1:1 ratio, in blocks of 4 to 6 (size randomly selected) and stratified by DIVA assessment (low, medium and high), using the DIVA key (Schults et al, 2022). Trial interventions will not be amendable to participant or staff blinding. Data collectors and trial statistician will be masked to group allocation.
Data collection
Quantitative data will be collected by the research nurses from the electronic medical record, and entered into a dedicated secure, online database (REDCap™; Research Electronic Data Capture) (Harris et al, 2009). Once enrolled in the study, the research nurses will follow-up participants daily (Monday to Friday only) until the PIVC is removed, and again at 48 hours post PIVC removal. The study manager will undertake quality checks and monitor 100% source data verification for: all data for the first patient, all consent forms, all primary endpoints and a random 5% of other data for all patients.
On enrolment and device insertion
Research nurses will collect consent forms, and demographic and clinical data to provide a description of the study participants (eg age, primary diagnosis, DIVA status) and device characteristics (eg, insertion location, gauge/length). Research nurses will assist with device insertion where possible, assess for primary outcome, and document number of insertion attempts, and number and designation of staff required for insertion.
On study
From Monday to Friday (not over weekends), research nurses will perform daily checks on enrolled patients to assess for secondary outcomes; current use; and any clinical, PIVC or treatment factors that have changed since recruitment.
On device removal, the research nurse will record the reason for removal, assess the PIVC insertion site (for device/site complication) and collect data from electronic records. In the event of device failure, the decision regarding a replacement device will be at the discretion of the treating team.
Data analysis
Analyses will follow the ‘intention to treat’ principle, with patients analysed in the group to which they were randomised (Newell, 1992). The patient is the unit of analysis, and there can be only one PIVC per participant. Baseline characteristics for each of the groups will be descriptively presented using frequency and percentage for categorical variables and mean and standard deviation (or median and interquartile range if appropriate) for continuous variables. The primary outcome, first-time insertion success, is to be analysed using a logistic regression model. Effect estimates are to be presented as odds ratios with corresponding 95% confidence intervals. Secondary outcomes with continuous data are to be analysed using linear regression, secondary outcomes with binary data using logistic regression, secondary outcomes with count data using Poisson regression, and secondary outcomes with time-to-event data using Cox proportional hazards models. Time-to-event data will be displayed using Kaplan-Meier survival curves by study groups. An a priori subgroup analysis, stratified by DIVA status, will be completed for primary and key secondary outcomes including number of insertion attempts, total PIVC insertion failure, PIVC dwell time, and PIVC post insertion failure. To test the sensitivity of results to changes in insertion technique a per protocol analysis will be conducted. A statistical analysis plan will be finalised before any analysis commences.
Costs are to be estimated by assessing the cost of the number of products used (for example PIVC, and insertion equipment such as ultrasound), and staff (for example nurse, medical officer), and staff time required for device insertion. Costs associated with sequelae (eg complications, replacement) are to be estimated based on reported health resources (including staff time) and utilisation at time of removal (as recorded in the electronic data collection tool). The difference between expected cost per patient between ultrasound-guided PIVC and landmark PIVC are to be estimated. Uncertainty in the estimated cost difference are to be explored using non-parametric techniques (bootstrapping: 1000 iterations with replacement).
Patient and public involvement
Satisfaction with existing PIVC insertion practices were sought from patients, parents, and clinical staff in a previous cohort study (Kleidon et al, 2019) undertaken at the Queensland Children's Hospital. Qualitative interviews associated with this project (Kleidon et al, 2019) unearthed parental frustration at the number of PIVC insertion attempts prior to reaching for assistive technology such as ultrasound, remarking that it seemed to be an afterthought. These findings, together with outcomes from an international survey (Schults et al, 2019) reporting a lack of planned escalation for difficult PIVC insertion, motivated this study (Schults et al, 2019). The overall results of the study will be communicated to study participants by sending a plain language summary to the provided email addresses.
Ethics and dissemination
This trial is registered with the Australian and New Zealand Clinical Trial Registry (ACTRN 12621000206820). Ethical approval for the trial has been obtained from Children's Health Queensland Hospital and Health Service Human Research Ethics Committee (HREC) (HREC/20/QCHQ/61248) University of Queensland HREC (UQ Ref No: 2022/HE002352) and Griffith University HREC (GU Ref No: 2021/042). This study is being performed in accordance with the ethical principles of the Declaration of Helsinki, ICH GCP for Guidance on Good Clinical Practice and with Australian Government NHMRC National Statement on Ethical Conduct in Research Involving Humans (World Medical Association, 2008; National Health and Medical Research Council et al, 2018).
A data safety management committee (DSMC) has been established to monitor the safety and progress of the trial. This compromises three committee members, a paediatric surgeon, a paediatric anaesthetist and a clinical triallist, who have been appointed by the research team based on their clinical experience in PIVCs. The DMSC will be forwarded copies of all serious adverse events (SAEs) reports as soon as they become available. The DSMC will report back to the chief investigator of the study if any further action is required.
Prospective written and informed consent is be obtained from the patient (where appropriate) and the patient's parent or guardian by the research nurse prior to enrolment in the study. Consent can be withdrawn. Withdrawal data are to be collected and included in the consolidated standards of reporting trials (CONSORT) diagram. Participants will not be identified by name, and confidentiality of all medical record information will be preserved. All participants' details will be entered in coded format and the confidentiality of the participant will be maintained unless disclosure is required by law.
The trial findings will be written up by the investigators and submitted to a peer-reviewed medical journal, preferably under an open-access format to permit free access to the published study. De-identified raw data will be stored within a data repository held at Children's Health Queensland. Dissemination will also include conference presentations.
Trial status
The trial commenced on 8 July 2021, and has now completed recruitment at time of publication.
Data statement
As the trial is ongoing, data generated and/or analysed will not yet be publicly available. On completion of the trial, the chief investigator (TMK) will make the data sets available on reasonable request, and following review of such a request by the original ethics committees.
Discussion
Globally, multiple PIVC insertion attempts and the sequalae of PIVC failure remains a common and unacceptable reality for many hospitalised children (Indarwati et al, 2020). Successful insertion of PIVC on first attempt does not always occur and rates vary among institutions (Goff et al, 2013; Bahl et al, 2021). International organisations (eg Infusion Nurses Society (Gorski et al, 2021), Emergency Nurses' Association of the USA (Crowley et al, 2012), the Australian Commission on Quality and Safety in Healthcare (Keogh et al, 2019), the Royal College of Nursing (Denton et al, 2016) and the Association of Anaesthetists of Great Britain and Ireland (Bodenham et al, 2016) all recommend the use of technology to improve PIVC first-attempt insertion success. Independent evaluation of the value and effectiveness of ultrasound guidance to insert PIVC in children of all levels of difficulty is necessary to ensure the safety and improved quality of life for our most vulnerable patients. If ultrasound-guided PIVC insertion proves to be a superior technique to traditional landmark, it is likely that patient satisfaction will improve and the costs associated with health care will be reduced.
KEY POINTS
- This is the first randomised controlled trial evaluating first-time insertion success of ultrasound-guided perioheral intravenous catheter (PIVC) insertion, in comparison with the standard landmark insertion technique, stratified by difficulty of PIVC insertion
- This is a pragmatic trial, reflective of the real-world non-expert vascular access insertion model, that is PIVCs are inserted by general clinical staff in a tertiary paediatric hospital, not via specialist or dedicated vascular access teams
- Consumer and clinician satisfaction with mode of insertion is assessed immediately following insertion using a 0–10 verbal rating scale
- The trial is held in a single-centre tertiary referral paediatric hospital, which will enhance internal validity, but may limit the generalisability of the findings
- The aim is to publish the results of this randomised controlled trial in a peer-reviewed journal
CPD reflective questions
- In your clinical practice, how important is PIVC first-time insertion success?
- What factors need to be considered before inserting a PIVC in a paediatric patient?
- In your healthcare facility what do you perceive to be the barriers and facilitators to implementing ultrasound-guided PIVC insertion?


