In response to increasing regulatory pressure to reduce central line-associated bloodstream infections, there has been a transition in the US away from the use of peripherally inserted central catheters (PICCs) and central venous catheters (CVCs) (Pathak et al, 2015; 2018). Midline catheters are associated with a lower incidence of device-related bloodstream infections than PICCs and CVCs and have gained popularity as alternative peripheral venous access devices for infusion therapies and frequent phlebotomy for up to 28 days (Chopra et al, 2015; Adams et al, 2016).
Previous studies reported that midline catheter occlusions occur at a rate of 2%–6%, but they did not evaluate management strategies for midline catheter occlusions (Campagna et al, 2018; Chopra et al, 2019; Tripathi et al, 2021). Therefore, limited research exists regarding the management of midline catheter occlusions.
Clear recommendations exist on the management of occlusions in different types of central catheter (PICCs and other CVCs) including mechanical occlusions caused by catheter malposition, chemical occlusions resulting from medication precipitates or lipid residues, and thrombotic occlusions caused by blood clots inside or at the tip of the catheter (Bolton, 2013; Marino 2013).
Tissue plasminogen activator (alteplase) is approved by the US Food and Drug Administration (US FDA) for restoring function to central venous access devices (CVADs) with a success rate of up to 87% after two 2 mg/2 ml doses instilled for 30–120 minutes (Deitcher et al, 2002). Despite the increased reliance on midline catheters, there is limited evidence on the use of alteplase for the restoration of midline catheter patency. The objective of this study was to describe the efficacy and safety of alteplase for the off-label management of midline catheter occlusions among hospitalised patients.
Methods
Study design and setting
This retrospective, descriptive study included adult patients who received at least one dose of alteplase (Cathflo Activase, Genentech USA) in a midline catheter between January 2015 and May 2018 within a multi-hospital health system that included one academic medical centre and seven community hospitals.
Alteplase is a tissue plasminogen activator (tPA) that exerts its thrombolytic effect by binding to fibrin in thrombotic clots and converting plasminogen to plasmin. Plasmin breaks down fibrin, leading to the degradation of the fibrin clot (Reed et al, 2022). The study was approved by the institutional review board of the study site with a waiver of informed consent.
Patient selection
Patients aged ≥18 years who had received at least one dose of alteplase in a midline catheter at any facility in the health system were included in this study.
The investigators retrieved reports from the electronic health records of patients who were admitted to hospital and had at least one dose of alteplase administered during the study period and cross referenced these with reports of hospital admissions that contained flowsheet documentation that a midline catheter was in place on the day when alteplase was administered.
For each of these admissions, investigators reviewed the chart to determine if alteplase had been administered into a midline catheter. Midline catheter insertion was confirmed using procedure notes for midline catheter insertion, nursing flowsheet documentation and imaging results to confirm the midline catheter tip position at or near the level of the axilla (Gorski et al, 2017).
Nursing assessments of midline catheter function charted at least once per 12-hour shift, notes from physicians and nurses and electronic medication administration records were reviewed for documentation of midline catheter malfunction and alteplase administration.
If the midline catheter was the only active vascular access device at the time of alteplase administration, it was assumed that alteplase was administered into that midline catheter in the absence of clear documentation of administration site.Where a patient had multiple active vascular access devices, alteplase administration for a catheter occlusion was excluded if a specific catheter malfunction had not been clearly documented.
Detailed methods for ascertaining lumen alteplase administration into double-lumen midline catheters are described in Supplementary Table 1 (available from the authors).
Occlusion events
Partial occlusion was defined as the ability to infuse fluids without the ability to aspirate or withdraw blood. Complete occlusion was defined as the inability to infuse fluids and aspirate blood (Bolton, 2013). When practitioners documented an infusion malfunction, the occlusion event was classified as complete occlusion.
The time of midline catheter occlusion was defined as the time of the first documentation of infusion or withdrawal malfunction, or the time of ordering alteplase for the midline catheter occlusion, whichever occurred first.
When two doses of alteplase had been administered into two different midline catheters, investigators assumed that two independent occlusion events had occurred. If an occlusion event resolved after the first alteplase administration and reoccurred more than 24 hours later, the second event was considered as new occlusion event. If an occlusion event failed to resolve or resolved temporarily for <24 hours after the first alteplase administration, a second alteplase dose was considered a continuation of treatment of the same occlusion event.
Primary outcome
The primary outcome was restoration of infusion function or withdrawal function to at least one lumen of the midline catheter treated with alteplase.
The restoration of infusion function in complete occlusions was defined as healthcare provider documentation of resolution or successful administration of intravenous fluids in the midline catheter treated with alteplase. The restoration of withdrawal function in partial or complete occlusions was defined as healthcare provider documentation of resolution or successful withdrawal of blood from the midline catheter treated with alteplase. If withdrawal function was restored, it was assumed that infusion function was also restored in the absence of explicit documentation.
Secondary outcomes
Secondary outcomes included the alteplase dose ordered, alteplase catheter dwell time, number of alteplase administrations per occlusion event, time between alteplase administrations if more than one dose was administered per occlusion event, number of midline catheter dwell days after alteplase administration and midline catheter removal or replacement after alteplase administration. If the midline catheter was not removed at hospital discharge, the date of hospital discharge was considered as the date of midline catheter removal.
Safety outcomes included bleeding, thromboembolic events, and new catheter-related bloodstream infections that occurred within 48 hours of the last dose of alteplase administered for each occlusion event.
Bleeding events were categorised as local bleeds around the midline catheter insertion site or other bleeds at sites distant from the midline catheter. Thromboembolic events included upper or lower extremity deep vein thrombosis, pulmonary embolism, or stroke confirmed by imaging studies. New catheter-related bloodstream infections were defined as blood cultures positive for an organism commonly associated with catheter-related bloodstream infections in the absence of other identifiable sources of infection.
Statistical analysis
Patient demographics, baseline characteristics and study outcomes were summarised as means with standard deviation (SD) or medians with interquartile range (IQR) for continuous variables and frequencies with percentages for categorical variables. Statistical analyses and data management were conducted using STATA v 15 (StataCorp, US).
Results
This study included 49 hospital admissions for 48 unique patients who received 50 midline catheters, experienced 53 midline catheter occlusion events, and received 59 alteplase administrations into midline catheters. After resolution of the first occlusion event, another occlusion event was detected in three midline catheters.
Patients
The analysis of patient demographics and baseline characteristics was performed for each hospital admission. The mean age was 64 (SD = 14) years, and 34 (69%) patients were admitted to an academic medical centre. Histories of ischaemic stroke were higher than expected and documented for nine (18%) patients. Before admission, 24 (49%) patients took antithrombotic therapy (Table 1).
Table 1. Patient demographics and baseline characteristics
| Characteristic | All included patients (n=49)* |
|---|---|
| Females, n (%) | 28 (57%) |
| Age in years, mean ± SD | 64 ± 14 |
| Weight in kg, mean ± SD | 86 ± 24 |
| Race, n (%) | |
| Caucasian/white | 29 (59%) |
| Black/African American | 18 (37%) |
| American Indian | 2 (4%) |
| Hospital type, n (%) | |
| Academic medical centre | 34 (69%) |
| Community hospital | 15 (31%) |
| Chronic kidney disease† | 11 (22%) |
| End-stage renal disease, on haemodialysis | 4 (8%) |
| Liver disease, n (%)‡ | 4 (8%) |
| Cancer, n (%)¶† | 4 (8%) |
| History of stroke† | |
| Ischaemic | 9 (18%) |
| Haemorrhagic | 0 (0%) |
| Anticoagulation therapy before hospital admission | 8 (16%) |
| Antiplatelet therapy before hospital admission | 18 (37%) |
* Data in this table describe 49 hospital admissions (for 48 patients) where alteplase was administered in a midline catheter. The analysis was performed for each hospital admission
† Extracted from physician notes in the electronic health record
‡ Defined as cirrhosis extracted from physician notes in the electronic health record or bilirubin >2 times, or alanine transaminase/aspartate transaminase/alkaline phosphatase >3 times upper normal limit
¶ Cancer being treated within 1 year before hospital admission
Midline catheters
Of the 50 midline catheters included in this analysis, 30 (60%) were double-lumen catheters, 35 (70%) were inserted in the basilic vein and 23 (46%) had a 5 Fr gauge size.
The overall median duration of midline catheter use was 7 days (IQR 4–12 days), which was within the duration of up to 29 days recommended by local institutional policy. Midline catheters were active for a median of 4 days (IQR 2–6 days) before alteplase administration (Table 2). Alteplase was first administered within 1 calendar day of insertion for 11 (22%) midline catheters. One of the included catheters was a PICC that could not be advanced past the axilla and was therefore included as a midline catheter.
Table 2. Midline catheter characteristics
| Characteristic | All midline catheters (π=50) |
|---|---|
| Number of lumens | |
| Single lumen, n (%) | 20 (40%) |
| Double lumen, n (%) | 30 (60%) |
| Insertion site | |
| Basilic vein, n (%) | 35 (70%) |
| Brachial vein, n (%) | 11 (22%) |
| Cephalic vein, n (%) | 4 (8%) |
| Catheter length in cm, mean ± SD* | 11.5 ± 2.5 |
| Catheter gauge size, n (%) | |
| 4 Fr | 13 (26%) |
| 5 Fr | 23 (46%) |
| Not reported | 14 (28%) |
| Catheter dwell days before the first alteplase administration†‡ | |
| Mean ± SD | 4.4 ± 3.3 |
| Median (IQR) | 4 (2 to 6) |
IQR=interquartile range, PICC=peripherally inserted central catheter
* Catheter length was reported for only 36 midline catheters
† If more than one occlusion event was identified in the same midline catheter, the time of the first alteplase administration for the first occlusion event was used for this calculation. If two alteplase doses were used to manage the same occlusion event, the time of the first alteplase administration was used for this calculation
‡ If the midline catheter had been placed at an outside facility before admission, the date of admission was considered to be the date of midline catheter insertion in the calculation of catheter dwell days before alteplase administration
¶ If the midline catheter was not removed at hospital discharge, the date of hospital discharge was considered as the date of midline catheter removal in the calculation of catheter dwell days after alteplase administration
Patient characteristics before each midline catheter occlusion
Of the 53 occlusion events included in this analysis, 41 (77%) occurred on an acute care floor. Bleeding was documented within 48 hours before alteplase administration in 14 (26%)
occlusion events; of these, two bleeding episodes occurred at the midline catheter insertion site before alteplase administration. Antithrombotic therapy was administered within the 24-hour period before alteplase administration in 40 (75%) occlusion events.
Positive blood cultures secondary to urinary tract infections were noted before two (4%) occlusion events. Medications with high occlusive risk had been given in five (9%) occlusion events in the 24 hours before alteplase administration (Table 3).
Table 3. Patient characteristics prior to each midline catheter occlusion
| Characteristic | Midline catheter occlusion event (n=53) |
|---|---|
| Care level, n (%) | |
| Acute care | 41 (77%) |
| Critical care | 7 (13%) |
| Skilled nursing facility/rehabilitation unit | 4 (8%) |
| Long-term continuing care | 1 (2%) |
| Bleeding episode prior to alteplase administration, n (%)* | 14 (26%) |
| Procedure performed, n (%)* | 8 (15%) |
| Antithrombotic therapy, n (%)† | 40 (75%) |
| Antiplatelet agents | 20 (38%) |
| Anticoagulants – VTE treatment or atrial fibrillation | 12 (23%) |
| Anticoagulants – VTE prophylaxis | 22 (42%) |
| Thrombolytic agents | 0 (0%) |
| NSAID therapy, n (%)†a | 0 (0%) |
| ESA therapy before alteplase administration, n (%) | 4 (8%) |
| Baseline laboratory values | |
| INR, mean ± SD‡b | 1.9 ± 1.0 |
| aPTT, mean ± SD‡c | 49 ± 10 |
| Haemoglobin (g/dl), mean ± SD¶d | 9.6 ± 1.9 |
| Haematocrit (%), mean ± SD ¶d | 30 ± 6 |
| Platelet count (x103 per µl), mean ± SD¶d | 205 ± 107 |
| Positive blood culture during the hospital admission before the first dose of alteplase administered for the occlusion event, n (%) | 2 (4%) |
| Sepsis treatment before the first dose of alteplase administered for the occlusion event, n (%) *e | 4 (8%) |
| High occlusive risk medication, n (%)†f | |
| Propofol | 1 (2%) |
| Calcium gluconate | 4 (8%) |
aPTT=activated partial thromboplastin time, ESA=erythropoietin stimulating agents, INR=international normalised ratio, NSAID=non-steroidal anti-inflammatory drug, VTE=venous thromboembolism
* Occurred within 48 hours before the first dose of alteplase was administered for the occlusion event
† Administered within 24 hours before the first dose of alteplase was administered for the occlusion event
‡ Last result within 24 hours before the first dose of alteplase was administered for the occlusion event
¶ Last result within 48 hours before the first dose of alteplase was administered for the occlusion event
a Not including aspirin for primary and secondary prevention of clotting disorders
b Baseline INR was available only before eight occlusion events
c Baseline aPTT was available before only four occlusion events
d Baseline haemoglobin, haematocrit and platelet count were available before 48 occlusion events
e Sepsis was documented in physician progress notes. Two patients were treated for sepsis with negative blood cultures: the first patient was admitted with septic shock secondary to Klebsiella urinary tract infection; the second patient was admitted with a urinary tract infection, sepsis and positive systemic inflammatory response syndrome
f The high occlusive risk medications and fluids that were reviewed and collected were total parenteral nutrition, lipid emulsions, propofol, clevedipine, phenytoin, calcium gluconate, calcium phosphate and calcium chloride. Only medications and fluids that were identified were reported
Primary outcome
Of the 53 occlusion events included in this analysis, 32 (60%) were partial occlusions, 17 (32%) were complete occlusions, and four (8%) could not be categorised into an occlusion type because of a lack of documentation.
In the 49 midline catheters with a known occlusion type, the median time from midline catheter occlusion until the administration of the first alteplase dose was 4.5 hours (IQR 2.5–14.7 hours).
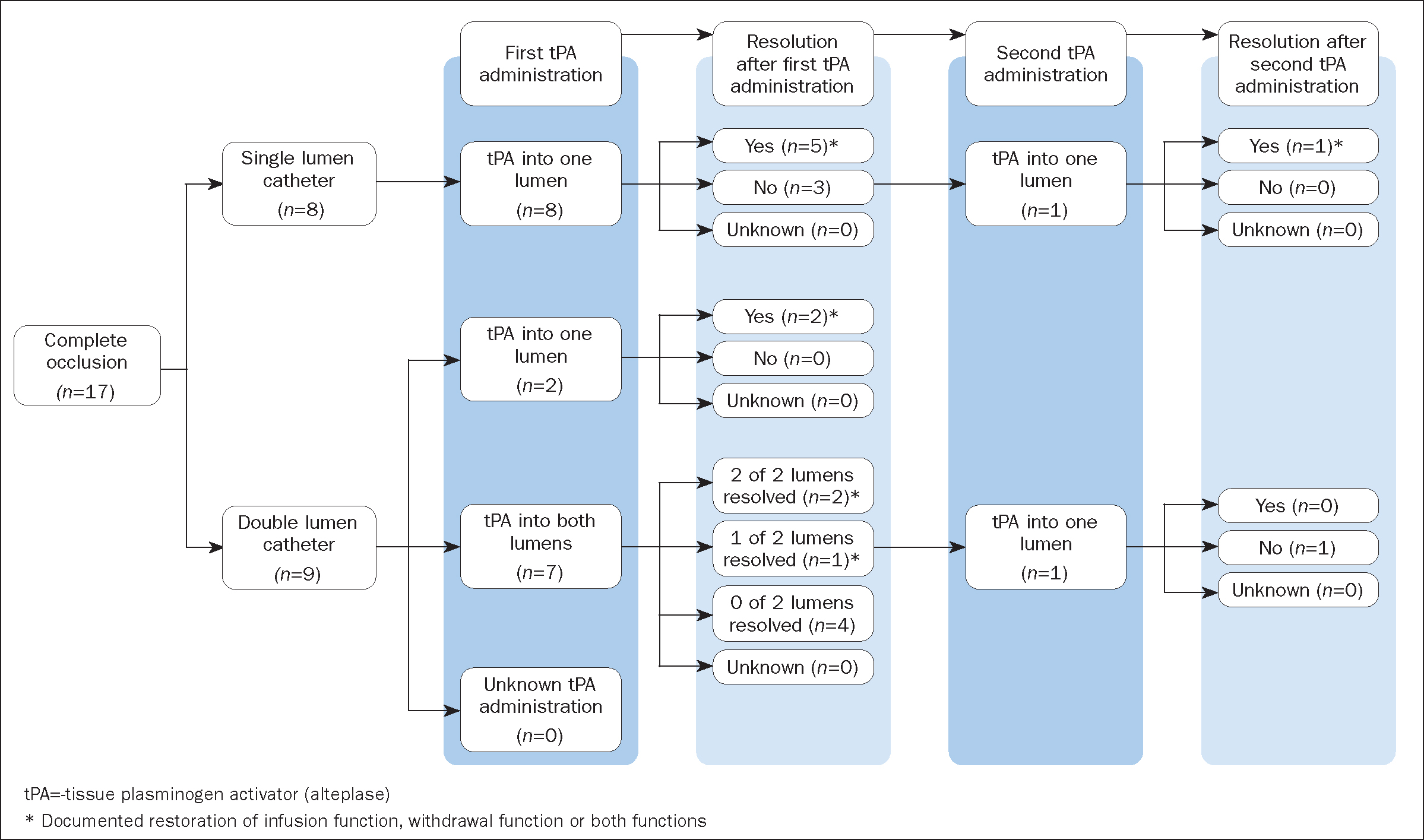
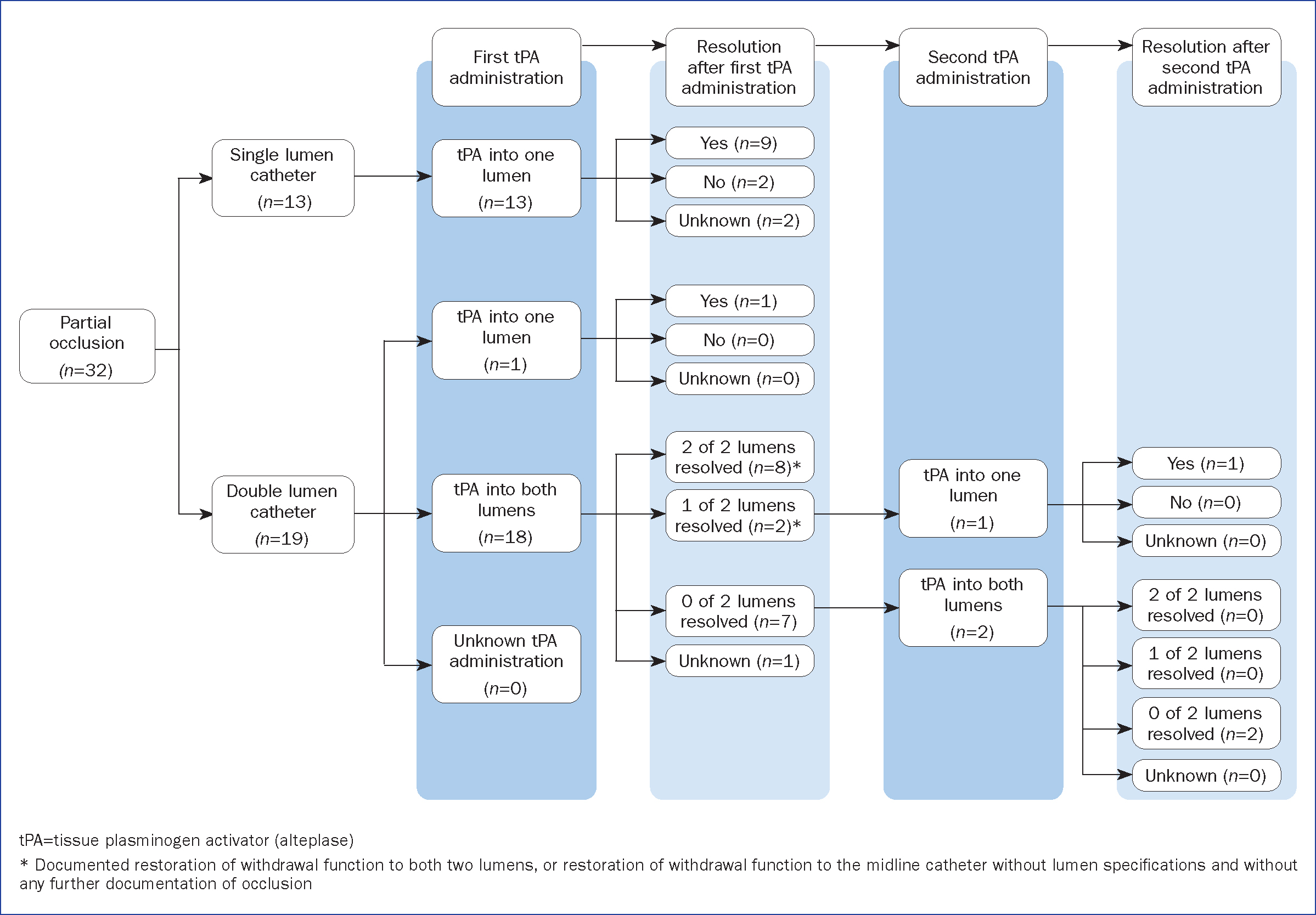
After the final alteplase administration, withdrawal function was restored in 25 of the 53 (47%; 95% CI [33%–61%]) occlusion events, and infusion function was restored in 11 of 17 (65%; 95% [CI 38%–86%]) complete occlusion events (Table 4).Restoration of infusion or withdrawal function after alteplase administration was documented in 58% (31 of 53) of occlusion events. After the first alteplase administration, any function (infusion function, withdrawal function or both infusion and withdrawal functions) was restored in 12 of 24 (50%) lumens with complete occlusions, and withdrawal function was restored in at least 28 of 50 (56%) lumens with partial occlusions. After the second alteplase administration, any function was restored in one of two (50%) lumens with complete occlusions, and withdrawal function was restored in one of five (20%) lumens with partial occlusions (Figure 1 and Figure 2). Resolution of lumen occlusion could not be evaluated for occlusions of an unknown type because of a lack of documentation (Supplementary Figure 1, available from the authors).
Table 4. Outcomes after alteplase administration in midline catheters
| Outcome* | All midline catheter occlusions (n=53) | Partial occlusion (n=32) | Complete occlusion (n=17) | Unknown occlusion type (n=4) |
|---|---|---|---|---|
| Efficacy outcomes | ||||
| Infusion function restored† | N/A | N/A | 11 (65%) | N/A |
| Withdrawal function restored† | 25 (47%) | 20 (63%) | 5 (29%) | N/A |
| Safety outcomes | ||||
| Local bleed around midline catheter insertion site‡ | 5 (9%) | 3 (9%) | 1 (6%) | 1 (25%) |
| Any other distant bleed‡ | 7 (13%) | 5 (16%) | 2 (12%) | 0 (0%) |
| Thromboembolic complicationsঠ| 1 (2%) | 1 (3%) | 0 (0%) | 0 (0%) |
| Catheter-related bloodstream infectionठ| 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
* If multiple doses of alteplase were used to manage the same occlusion event, the final outcome after the second (the last) alteplase administration was reported
† Restoration of function to at least one midline catheter lumen
‡ Occurred within 48 hours of the last alteplase dose administered for the occlusion event
¶ Thromboembolic complications were defined as upper/lower extremity deep vein thrombosis, pulmonary embolism or stroke that was confirmed by imaging studies
§ New catheter-related bloodstream infections were defined as blood cultures positive for an organism commonly associated with catheter-related bloodstream infections in the absence of other identifiable sources of infection
Secondary outcomes
Of the 59 alteplase administrations, the doses ordered were 1 mg for 38 (64%) orders, 2 mg for 20 (34%) orders and 0.5 mg for one (2%). However, the actual volume instilled in the midline catheter could not be determined.
Dwell time was documented for only six alteplase administrations (2 hours for five administrations and 1 hour for the other one) for five occlusion events (four complete and one partial), of which one complete occlusion event resolved after alteplase administration. A single alteplase dose was administered for 47 occlusion events, and two doses were administered for six occlusion events, with a median of 24 hours (IQR 7–26 hours) between the two doses (Supplementary Figure 2, available from the authors). Midline catheters remained in place for a median of 2.5 days (IQR 1–6 days) after alteplase administration. Of 50 midline catheters, 30 (60%) were maintained until discharge, three (6%) were removed because they were no longer indicated and 17 (34%) were replaced because of malfunction (Table 2). As a replacement for the midline catheter, one patient received a Hickman central catheter.
Mild local bleeding around the midline catheter was documented after five of the 53 (9%) occlusion events had been treated with alteplase and did not result in haemoglobin drop or blood transfusions. Of the patients with local bleeds, two were receiving prior dual antiplatelet therapy and venous thromboembolism prophylaxis, and one had prior ecchymosis and was receiving warfarin therapy. Distant, systemic or local bleeds documented in seven of the 53 (13%) occlusion events were attributed to recent procedures or patients’ underlying comorbidities and were not related to alteplase administration (Table 4).
One patient was diagnosed with an acute thrombosis of the right cephalic vein and a laminating thrombus adhering to the midline catheter resulting in arm immobility and was evident on duplex venous ultrasound performed on the day following alteplase, that is day 6 of the midline catheter. However, the patient was in a hypercoagulable state secondary to endometrial cancer and obesity, with recent diagnoses of bilateral pulmonary embolism, acute ischaemic stroke, extensive bilateral lower extremity deep vein thrombosis, myocardial infarction and coronary artery disease. No new catheter-related bloodstream infections were detected after alteplase administration (Table 4).
Discussion
Maintenance using appropriate flushing and locking techniques is essential to prevent midline catheter occlusions. Appropriate flushing involves using a pulsatile flow to flush 10 ml of normal saline before and after medication administration and blood sampling. A 20 ml volume of normal saline is recommended for flushing after infusion of viscous products such as lipid-based solutions and blood components. Appropriate locking involves closing the clamps and injecting 1.5 ml of normal saline into the midline catheter for the period when it is not being used (Goossens, 2015).
Similarly and in accordance with the Infusion Therapy Standards of Practice for vascular access devices, the midline catheter maintenance protocol at the authors’ institution stipulates that a 10 ml normal saline flush is used before and after medication administration, a 20 ml normal saline flush is used after blood withdrawals and a 10 ml normal saline flush is used every 8 hours for saline-locked midline catheters (Goossens, 2015; Gorski et al, 2017; 2021).
If these preventive techniques fail, midline catheter occlusions may require rapid treatment to maintain intravenous access. When choosing the optimal treatment (alteplase or catheter replacement), the clinical team should consider several factors, including effectiveness, cost (of medication and/or equipment), patient discomfort, staff time and availability, complications (infection, bleeding or missed medication administrations) and anatomical complexities (difficult intravenous access).
This study described the effectiveness of alteplase using real-world data. After alteplase administration, withdrawal function was restored in 63% of midline catheter partial occlusions and in 29% of complete occlusions, and infusion function was restored in 65% of complete occlusions.
Alteplase is approved in the USA for the restoration of function to CVADs (US FDA, 2001). In the two pivotal prospective phase III clinical trials of alteplase for dysfunctional CVADs, catheter function restoration was achieved in 75% of the patients after the first alteplase dose and in 85% after two doses. No cases of intracranial haemorrhage or embolic events were observed. Three cases of major haemorrhage and three cases of thrombosis were reported but not attributed to alteplase treatment. Sepsis or fever was documented in five patients, and only one fever event was related to alteplase (Semba et al, 2002).
In the present study of 53 midline catheter occlusion events, the majority of patients received only one alteplase dose, and adequate success rates were observed. Results of this study suggested that alteplase had a safety profile comparable to that seen in CVAD trials, and patients who were receiving multiple antithrombotic agents appeared to have a higher risk of local bleeding around the midline catheter after alteplase administration.
In a retrospective study that evaluated alteplase infusion versus dwell for clearance of central catheter partial occlusions in critically ill paediatric patients, nine catheters were PICCs or CVCs but not in a central position at the time of alteplase administration and were considered midline catheters. All midline catheter partial occlusions resolved after an alteplase dwell of 1 mg/ml over 0.5–2 hours (n=2) or alteplase infusions of 0.1 mg/kg (up to 2 mg/dose) over 3 hours (n=7) (Ragsdale et al, 2014). Although these nine catheters were intended to be CVADs, the findings suggest a possible role for alteplase in clearing partial occlusions in catheters not in a central position.
In a recently published prospective study conducted at a rural community hospital, patency was restored in 97% (109/112) of midline catheters treated with alteplase for a presumed thrombotic occlusion. That study included patients who had a midline catheter placed by vascular access specialists, and nurses were provided with an institutional protocol to assess the reason for occlusion and administer alteplase (Hawes, 2020). After a review of this data, the 8th edition Infusion Therapy Standards of Practice (Gorski et al, 2021) designated the use of alteplase for the management of thrombotic occlusions in midline catheters as off label pending further investigation beyond the single study conducted by Hawes (2020).
The present study provides additional insight into the effectiveness of alteplase at an academic medical centre and multiple community hospitals with heterogenous practices of midline catheter placement and management. Restoration of function after alteplase administration was documented in 58% of occlusion events in this study, and this lower response may be because there is no standardised protocol for patient selection and alteplase dosing and dwell time. Additionally, the authors characterised occlusion types as partial or total. and described the restoration of either infusion function or withdrawal function.
Future studies could improve upon the present study and that by Hawes (2020) by: confirming occlusion with ultrasound imaging before alteplase administration; using a protocol for alteplase dosing and dwell time; and providing details on occlusion type and restoration type.
Limitations
Midline catheters are peripheral venous access devices that terminate in a smaller vein than CVADs. Recently, Qin et al (2019) proposed standardised nomenclature to differentiate between long peripheral catheters and midline catheters based on catheter length (6–15 cm versus 15–25 cm), catheter tip extension (distal to axilla versus infra/supraclavicular region) and insertion technique (direct versus modified Seldinger). Catheters included in the present study were labelled as midline catheters in the electronic health record, and further differentiation was not possible because of limitations within clinical documentation. Differentiating between long peripheral catheters and midline catheters should be considered in future studies evaluating alteplase for catheter occlusion.
In this study, the aetiology of withdrawal malfunction events such as small vein collapse, mechanical obstruction or thrombotic occlusion could not be determined. However, chemical occlusions were unlikely because medications and lipid solutions with a high precipitating potential had been administered through the midline catheter in only five (9%) occlusion events within the 24 hours before alteplase administration. Approximately 22% of midline catheter occlusions occurred within 1 day of catheter insertion and could have resulted from malposition or mechanical obstruction. The mechanism by which these occlusions occurred could not be described because of the lack of information in the electronic health record.
This study did not include a control group of midline catheter occlusions that were not treated with alteplase.
Because of the lack of an institutional protocol, this retrospective study was unable to identify criteria used by bedside clinicians to determine which midline catheter occlusions were or were not treated with alteplase.
Some midline catheters were placed or removed at an outside facility, and the exact placement or removal date could not be confirmed. Some midline catheters at one hospital were placed by a dedicated vascular access team, but the other hospitals did not have a vascular access team for midline catheter placement during the study period. Because of inconsistencies in clinical documentation practice, occlusion type was unknown for 8% (4/53) of occlusion events, and outcomes following alteplase were unknown for 13% (7/53) of occlusion events.
Alteplase dwell time and the dose administered into each lumen was not documented for most patients. The approved alteplase dose for CVAD occlusions is 2 mg/2 ml for patients weighing 30 kg and above or 110% of the internal catheter lumen volume for patients weighing <30 kg without exceeding 2 mg/2 ml (US FDA, 2001; Deitcher et al, 2002; Blaney et al, 2006).
Priming volumes of single- and double-lumen PICCs range between 0.6 ml and 1.1 ml, and a 2 mg/2 ml dose of alteplase would provide additional extraluminal volume to treat fibrin tails (Navilyst Medical, 2012). For midline catheters, priming volumes are much smaller and range between 0.13 ml and 0.6 ml; therefore, a 1 mg/1 ml dose of alteplase may be reasonable for midline catheters, but optimal dosing requires further study (Bard Access Systems, 2015; Hawes, 2020; Navilyst Medical, 2020).
Conclusion
Alteplase administration into midline catheters resolved most (65%) infusion malfunctions and half (47%) of withdrawal malfunctions. Most (66%) midline catheters were salvaged until hospital discharge or end of therapy.
Local bleeding after alteplase was infrequent and mild. The proportion of midline catheter occlusions that resolved with off-label alteplase in this study was similar to that reported after one dose of alteplase for CVAD occlusions in previous clinical trials.
The results of this study warrant further prospective studies to evaluate the safety, efficacy and cost-effectiveness of alteplase in the treatment of thrombotic midline catheter occlusions.
KEY POINTS
- Midline catheters have gained popularity as peripheral venous access devices, but there is limited data on the use of alteplase for managing midline catheter occlusions
- Most midline catheter occlusions treated with alteplase demonstrated restoration of infusion or withdrawal function
- Alteplase appears to be a safe treatment to restore midline catheter occlusion, and bleeding after alteplase was infrequent and mild
- Further prospective studies are needed to evaluate alteplase for the treatment of thrombotic midline catheter occlusions
CPD reflective questions
- How frequently are midline catheters used in your place of work and how often do they become occluded?
- What are the consequences of losing vascular access for a hospitalised patient?
- How are midline catheter occlusions commonly managed at your place of work and how quickly can you get a midline catheter replaced without compromising patient care?


