There has been a growing consensus that it is now important to protect children and young people by facilitating their participation in clinical trials rather than ‘protecting them from’ participation. Undoubtedly the inclusion of children and young people in clinical trials is ethically complex and has additional requirements to that of adult trials; however, establishing further medications and treatments that are safe and efficacious is essential.
Why are clinical trials required for children and young people?
It is widely recognised that due to developmental and physiological differences (Joseph et al, 2015; Naka et al, 2017) children and young people's participation in clinical trials is integral to the development of new medications that are both safe and efficacious (Cain and McGuinness, 2005; Hunfeld and Passchier, 2012; Staphorst et al, 2015). There is a balance to be achieved between ensuring the paediatric participant is not harmed during individual clinical trial participation and ensuring that the paediatric population is not harmed due to insufficient evidence for prescribing (Sammons and Starkey, 2016). This is particularly pertinent for children and young people with chronic conditions, with increased survival rates facilitated by modern medicine meaning they are living into adulthood, often without prospect of curative treatment (Rao et al, 2007; Moreira et al, 2013). Therefore, further research potentially offering improved care, treatment and quality of life is required (Pagano-Therrien and Sullivan-Bolyai, 2017).
Off-label medication use in children and young people
Off-label prescribing is common practice in paediatric healthcare (McLay et al, 2006) due to the historical lack of clinical trials assessing medications for use in the paediatric population. This has been caused by children being deemed too vulnerable to participate in research and profits for pharmaceutical companies being small or nonexistent (Medical Research Council, 2004). Although an EU regulation in 2007 (regulation EC No 1901/2006) sought to improve this by ensuring new clinical trials for medication were also trialled in the paediatric population, this is only applicable to the development of new medications and does not apply to the plethora that already exist. Studies across different countries have found varying prevalence of off-label/unlicensed prescribing for paediatrics: 22% in Bratislava, 28.3% in Australia, 32.2% in Portugal, 39.7% in France 42.1% in Palestine and 46.5% in Canada (Khdour et al, 2011; Slažneva et al, 2012; Ribeiro et al, 2013; Czarniak et al, 2015; Joret-Descout et al, 2015; Corny et al, 2016). Research therefore demonstrates that this is an international issue.
Although many drugs used off label have a strong scientific basis (Corny et al, 2016), without robust trials, crucial aspects of prescribing, such as pharmacokinetics, are unknown (Ginsberg et al, 2002; Anderson et al, 2006). Understandably, while McLay et al (2006) highlight that the majority (90%) of paediatricians they surveyed knowingly prescribe off-label medications, only 70% also had safety concerns. Thus while there are inherent risks and ethical issues within running paediatric clinical trials there is also an ethical responsibility to ensure children and young people are able to receive the available treatment (Medical Research Council, 2004).
Phases of clinical trials
There are four phases of clinical trials in humans. Table 1 was formulated using terminology from the National Institutes of Health (2016). Children may be involved at any stage; however, they are only likely to be involved at phase I if the disease does not occur in adults, if to give the healthy adult volunteer the medication would cause harm or if the child or young person has a condition for which curative care is no longer possible (Naka et al, 2017).
| Phase of clinical trial | Aims of the clinical trial | Number of participants |
|---|---|---|
| I |
|
20−80 |
| II |
|
100−300 |
| III |
|
1000−3000 |
| IV |
|
Not defined |
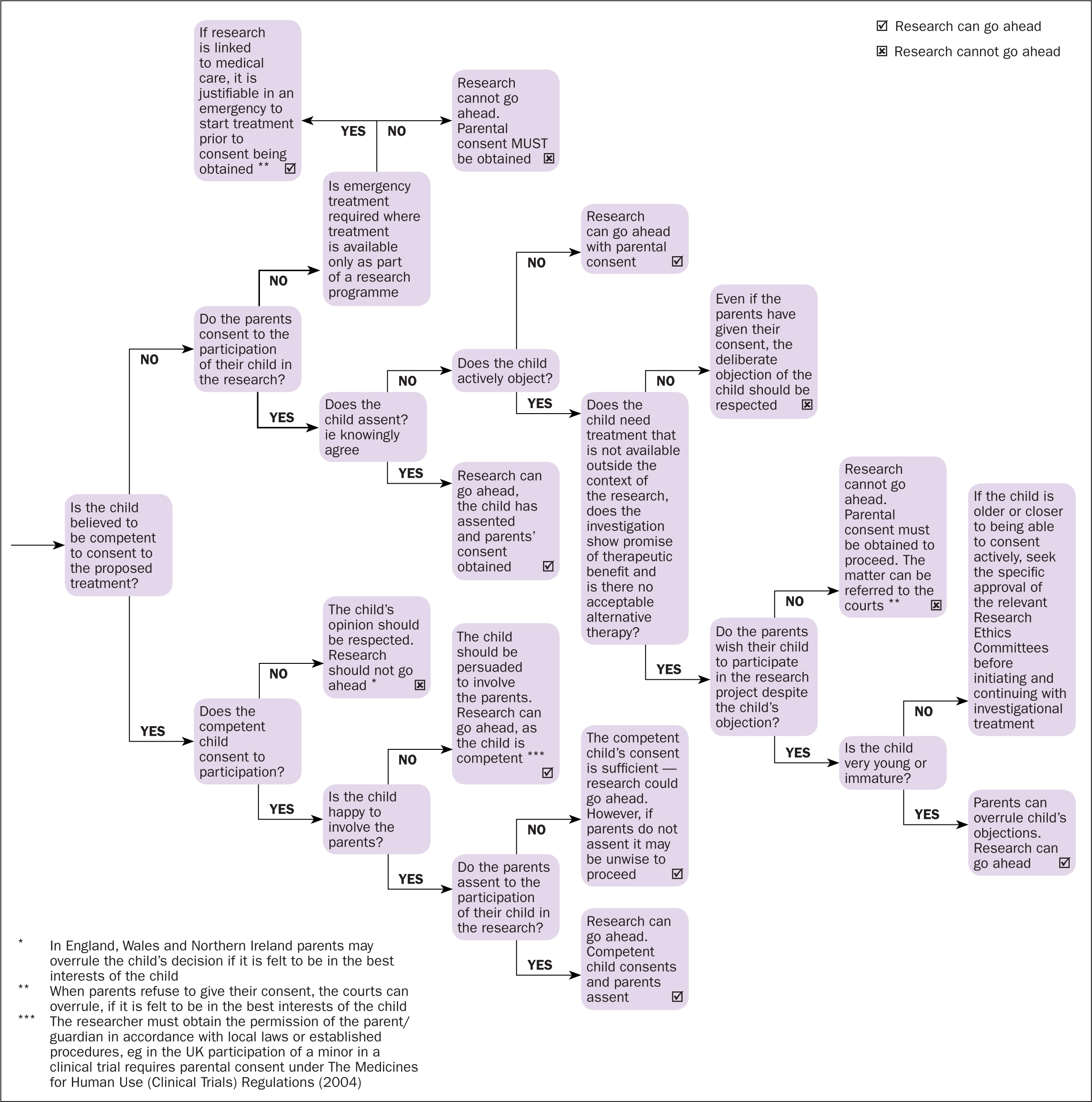
Consent and assent
Good clinical practice in medical research with children and young people calls for robust consent and assent processes ensuring ethical governance frameworks are adhered to (Royal College of Nursing (RCN), 2011).
According to the Health Research Authority (2019), ‘consent is a legally defined decision’ to participate in research. Assent, however, is a less well defined term that is usually used to denote that someone who is not legally able to provide consent for themselves is willing to participate (Grady et al, 2014; Madden et al, 2016; Health Research Authority, 2019).
Good clinical practice (World Health Organization, 2002) aims to ensure that the prospective participant understands what the study is trying to achieve, the risks of the study and what participation will entail (Sammon and Starkey, 2016). Due to clinical guidelines and statutory recommendations it is a legal and ethical imperative that informed consent (and where applicable assent) is sought (Lepola et al, 2016). This would occur if the child or young person is under 16, and may extend to 18 depending on the procedures involved in the trial, ethical safeguards and sponsor's country or origin, or if the young person lacks mental capacity. Assent from the child or young person should be sought from age 7 (Lepola et al, 2016; Naka et al, 2017; Health Research Authority, 2019). These standards differ from country to country and should be checked on an individual basis.
Although some children and young people choose to participate in research for altruistic motives, this is often more complicated and takes into account a range of inter-related factors (Barned et al, 2018). The same is true of parents, but is further complicated by the need to be a ‘good parent’ (Levine et al, 2015). The literature shows that, while children and young people mostly understand the study as a whole, less than half have a sound understanding of the procedures involved (Hunfeld and Passchier, 2012; Pagano-Therrien and Sullivan-Bolyai, 2017). Of concern is that many children and young people found that, with hindsight, they would choose not to participate if they were to be offered the study again (Staphorst et al, 2015). This may be due to the language used in information materials (Friedman et al, 2014) or misconceptions held about paediatric research in the wider population (Tromp and van de Vathorst, 2019) (see Figure 1).
Motivation
There is some evidence of the practical benefits of taking part in medical research at an individual level. For example, some participants appreciated the additional monitoring provided (Zvonareva et al, 2015) while others were hopeful of direct clinical benefit (Moore, 2001) or gained access to an otherwise unavailable drug (Naka et al, 2017). Further potential benefits are less tangible, such as maintaining ‘hope’ or ascertaining ‘power’ over the disease (Pinto et al, 2018).
The Medical Research Council guidance (2004) clearly states that for consent to be informed researchers must have discussed with the potential participants any possible direct benefit; however, in light of hope this continues to be confused. There is an established evidence base on therapeutic misconception (Oberman and Frader, 2003; Woods et al, 2014) which shows that young people, in particular, continue to be confused between research and clinical care (Hein et al, 2015), highlighting the importance for researchers of not overstating possible benefits, which can potentially influence motivation to participate.
Risk and burden
Achieving an acceptable balance of risk and benefit is thought to be a guiding ethical principle (Medical Research Council, 2004) yet risk is often considered at an individual level and judged against benefit at a population level. This is further compounded by the inconsistency of risk assessment at institutional review boards (Van Hoof et al, 2018). Although risk includes the likelihood of causing harm (physically or psychologically) burden centres on the child's and parents' burdens experienced during participation. This becomes difficult as although risk may be assessed as minor, such as repeated venepuncture or a nasogastric tube (Sammons and Starkey, 2016), the physical discomfort caused by this may result in significant burden (Staphorst et al, 2015; Barned et al, 2018). Thus, due to the recognition that the language used in discussion of risk can influence consent (Sammons and Starkey, 2016), it is especially important to be cognisant of burden as a separate concept. Both Barned et al (2018) and Van Hoof et al (2018) highlight the subjectivity of burden in that what some participants find burdensome others do not, but also that burden is open to misestimating by researchers and clinicians. Van Hoof et al (2018) also point out the burden of paediatric clinical trial participation faced by parents, this is echoed by Franson et al (2019).
Children and young people's voice
There is little literature or evidence on children and young people's subjective experience of clinical trial participation (Staphorst et al, 2015; Crane et al, 2018). A potential aid to the design of participant-friendly studies would be to involve service users in the trial design. Although this is widely accepted as good practice in general research with children and young people (Medical Research Council, 2004; RCN, 2011) it is not always explicit in clinical trial design.
Summary
Children and young people's participation is necessary and unavoidable to develop medications that are safe and efficacious with robust evidence for age groups, diseases, routes and doses. Consent of the parent and assent of the participant are not always aligned and should both be given full respect and consideration. There is a paucity of literature surrounding the child or young person's experience of participation in clinical trials (Crane et al, 2018) although it is acknowledged that each experience is unique to the individual (Barned et al, 2018) without further research it is unclear whether a commonality of experience exists.

