According to the British Lung Foundation, an estimated 1.2 million people are living with diagnosed COPD (BLF, 2017a). In terms of diagnoses, this makes COPD the second most common lung disease in the UK after asthma (BLF, 2017a). Around 2% of the whole population—4.5% of all people aged over 40—live with diagnosed COPD (BLF, 2017a). These numbers are steadily increasing year on year. Moreover, according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD), COPD is a major cause of chronic morbidity and mortality around the world, and is projected to be the third leading cause of death globally by 2020 (GOLD, 2019).
Malnutrition is a common problem in patients with COPD; it is suggested that 35% of hospitalised patients with the condition (Steer et al, 2010) and 22% of outpatients (Collins et al, 2018) are at risk of developing malnutrition. Inevitably, this will lead to an increase in hospitalisation (Hoong et al, 2017). The direct cost of malnutrition to the NHS is estimated to be £19 billion per year in England alone (Elia, 2015), which equates to £90 million per clinical commissioning group (CCG) (Kominek et al, 2017).
Consequences of malnutrition in COPD
Malnutrition is associated with a wide range of serious and potentially life-threatening conditions and complications, particularly in patients with conditions such as COPD (Box 1).
Box 1.The consequences of malnutrition, with a focus on chronic obstructive pulmonary disease
- Increased length of hospital stay
- Poor lung function secondary to decreased muscle strength
- Rapid deterioration in lung function
- Decrease in exercise capacity
- Inability to shop, cook and carry out activities of daily living
- Increased fatigue and apathy, which in turn delay recovery, exacerbate anorexia and increase convalescence time
- Increased risk of pressure ulcers, delayed wound healing and infection
- Reduced ability to cough, which may predispose the patient to chest infections and pneumonia
- Heart failure
- Increased risk of falls
- Poor renal function, leading to over- or under-hydration
- Increased social isolation
- Increased risk of depression
Adapted from Barker et al (2011); Bostock-Cox (2017); British Association of Parenteral and Enteral Nutrition (2018)
Patients with advanced COPD are often in a state of undernutrition, which has been referred to as ‘pulmonary cachexia’ (Itoh et al, 2013). Indeed, it has been reported that 25–40% of patients with COPD are in an undernourished state (Itoh et al, 2013).
Although there is no official definition of ‘pulmonary cachexia’, cachexia has been defined as ‘a complex metabolic syndrome associated with an underlying illness, characterised by loss of muscle with or without loss of fat mass’ (Argilés et al, 2011). Cachexia and muscle wasting are common features of COPD, which, although partly reversible, adversely affect disease progression and prognosis if not managed correctly (Sanders et al, 2016).
Causes of weight loss and malnutrition in COPD
The causes of weight loss and malnutrition in patients with COPD are complex and mainly attributed to skeletal muscle wasting (Passey et al, 2016).
Patients with COPD have greater resting energy expenditure and protein turnover than healthy individuals (Kao et al, 2011). Moreover, for many people with COPD, the sheer increased effort of breathing means the physical effort required to eat is increased which will have a significant impact on the ability to finish meals (Bostock-Cox, 2017). Dysphagia secondary to dyspnoea, when combined with chronic mucus production, mouth breathing and coughing, also contributes to a poor nutritional intake (Gandy, 2014).
Sarcopenia (the degenerative loss of skeletal muscle mass, quality and strength) is thought to affect 15% of patients with stable COPD, impairing their functional status and health (Jones et al, 2015). Physical issues associated with sarcopenia include a more severe dyspnoea scale score and lower exercise tolerance (Byun et al, 2017). There is also interest in the role of sarcopenia and obesity in the pathogenesis of undernutrition in COPD (Koo et al, 2014). It is important to be aware that there may be evidence of significant muscle wasting (in particular, respiratory muscle wasting) in a person who is obese, which indicates malnutrition (O'Donnell et al, 2014).
It is also important to understand the pathology of muscle wasting at a cellular level, since the inherent adaptability of muscle tissue offers nutritional therapeutic options to reverse or delay disease progression (Passey et al, 2016). Oxidative stress is implicated in the pathogenesis of respiratory diseases, including COPD (Thomson, 2018), and is characterised by the release of free radicals, resulting in cellular degeneration that damages cell membranes (Ohira et al, 2008). In patients with COPD, oxidative stress is thought to exacerbate the loss of lean muscle mass, so promotes cachexia (Rawal and Yadav, 2015).
COPD is also considered to be a systemic inflammatory disease, characterised by the production of inflammatory cytokines, including interleukin-6, interleukin-8 and tumour necrosis factor-alpha (Itoh et al, 2013). Such inflammatory mediators are thought to play a significant role in the pathogenesis of undernutrition in COPD (King, 2015).
More recently, there has been a focus on the role of alpha-1-antitrypsin deficiency, the most common known genetic cause of COPD, which is thought to have a significant impact on the regulation of systemic glucose and lipid metabolism in the pathogenesis of nutritional disorders associated with the condition (Beiko et al, 2017).
It is well documented that respiratory musculature can undergo structural and metabolic changes (Gea et al, 2015), and a key feature of musculature adaptation in COPD is a change in fibre composition from type I oxidative fibres to type II fibres (type IIx glycolytic and type IIa intermediate oxidative/glycolytic fibres) (Natanek et al, 2013; Sanders et al 2016). In simple terms, type I fibres are resistant to fatigue because of their ability to metabolise nutrients, whereas type II fibres have a much slower metabolism and tire easily, which has been shown to contribute to undernutrition in those with COPD (Polla et al, 2004).
All of the aforementioned potential causes of malnutrition in COPD are linked to raised protein metabolism (Remels et al, 2013). Protein is essential for growth, repair and immunity, and proteins help to maintain muscle mass, including those required for respiratory effort (Hodson, 2017). Protein requirements increase with age and also with disease processes such as COPD (Deutz et al, 2014). The European Society for Clinical Nutrition and Metabolism advises that older adults (aged >65 years) with long-term conditions such as COPD require 1.2–1.5 g of protein per kg of body weight per day, compared with 1.0–1.2 g protein/kg body weight/day for healthy older adults (Deutz et al, 2014). Patients with infective exacerbation of COPD require 1.5 g protein/kg body weight/day (Vermeeren et al, 1997).
Importance of screening
The assessment of body composition in COPD is important since weight loss and muscular wasting are responsible for low exercise capacity in patients with the condition (Gologanu et al, 2014). Furthermore, low BMI and fat-free mass (FFM) are important prognostic factors for the disease process, representing different aspects of nutritional abnormalities in COPD (Ischaki et al, 2007; Lainscak et al, 2011). BMI is relatively easy to measure and is part of the BODE (BMI, obstruction, dyspnoea, and exercise capacity) index, which is the most widely used tool to predict mortality in COPD patients (Guo et al, 2016). FFM is significantly correlated with exercise capacity, dyspnoea, respiratory muscle function and pulmonary function, and may therefore be a useful predictor of COPD severity (Luo et al, 2016).
It has been suggested that the use of BMI to assess an individual's nutritional status, in particular the levels of intra-abdominal fat, is less useful than the use of waist:height ratio (Ashwell and Gibson, 2009). Furthermore, BMI does not reflect age-related changes in height, posture or fat/muscle mass in the elderly and is therefore considered to be a less reliable marker of nutritional status in this population compared with younger age groups (Kimyagarov et al, 2010). In addition, a high BMI does not rule out malnutrition (Kelly, 2007). Consequently, it has been argued that FFM may be the more reliable indicator of malnutrition in those with COPD (King, 2015). Despite this, patients with COPD with an initial BMI of <20 kg/m2 or weight loss during 1 year of follow-up have a higher risk of acute exacerbations and mortality than those with a BMI of ≥20 kg/m2 or no weight loss over this follow-up period (Rawal et al, 2015). Therefore, it is likely that BMI and FFM are both important indicators of malnutrition in COPD.
The National Institute of Health and Care Excellence (NICE, 2018) guidelines on the diagnosis and management of COPD recommend that BMI is calculated at the time of initial diagnostic evaluation and that, from diagnosis onwards, a low BMI should be considered when discussing prognosis and treatment decisions with patients with stable COPD, since it is individually associated with prognosis (NICE, 2018). Patients with mild, moderate or severe COPD (stages 1–3) should have their BMI assessed at least annually, while those with very severe COPD (stage 4) should have it measured at least twice per year (NICE, 2018).
The NICE (2018) guidelines also highlight the importance of nutritional screening and nutritional support in COPD. Given the seriousness of the potential consequences of malnutrition (Box 1), it is vital that all clinicians understand the importance of nutritional screening for those with COPD and manage malnutrition to maximise nutritional status (Shepherd, 2010). Nutritional screening should be a quick, simple procedure carried out at the first point of patient contact (Shepherd, 2010).
The Malnutrition Pathway, devised in 2012 and updated in 2017 (Holdoway, 2017), provides recommendations for managing adult malnutrition in primary care. Although this tool is helpful in the assessment and management of malnutrition in general terms, it has been felt that it may not fully address the nutritional needs of those with COPD (Kominek et al, 2017). Therefore, in 2016, a multiprofessional panel with expertise and interest in malnutrition and COPD worked together to update the 2011 version of the Respiratory Healthcare Professionals Nutritional Guidelines for COPD Patients, which resulted in the publication of Managing Malnutrition in COPD (www.malnutritionpathway.co.uk/library/mm_copd.pdf), a new set of guidelines, which have been endorsed by NICE (Banner et al, 2016). Within the goals of managing malnutrition in COPD, these guidelines advise that it may be appropriate to aim for an increase in body weight and FFM in individuals with stable COPD; in those who are malnourished, a 2 kg increase is suggested as a threshold at which functional improvements are observed (Banner et al, 2016).
Role of oral nutritional supplements
Weight loss in COPD was thought to be an inevitable part of disease progression, but there is now convincing evidence that weight loss is an independent determinant of survival, supporting the need for weight maintenance in patient care (Sanders et al, 2016).
The Managing Malnutrition in COPD tool starts by calculating the risks of malnutrition using the Malnutrition Universal Screening Tool (MUST) (British Association for Parenteral and Enteral Nutrition (BAPEN), 2011; Banner et al, 2016). An important recommendation of the Managing Malnutrition in COPD guidelines is that patients whose BMI is <20 kg/m2 or who are at high risk of malnutrition should be given oral nutritional supplements (ONS) to increase their total calorific intake and promote weight gain (Figure 1) (Banner et al, 2016). Dietary advice should be used alongside ONS only where indicated (ie when BMI is <20 kg/m2 or in high-risk individuals (Banner et al, 2016), and in those whose BMI is high (≥25 kg/m2) (NICE, 2018). If there is no improvement or more specialist support is required, patients should be referred to a dietitian (Banner et al, 2016).
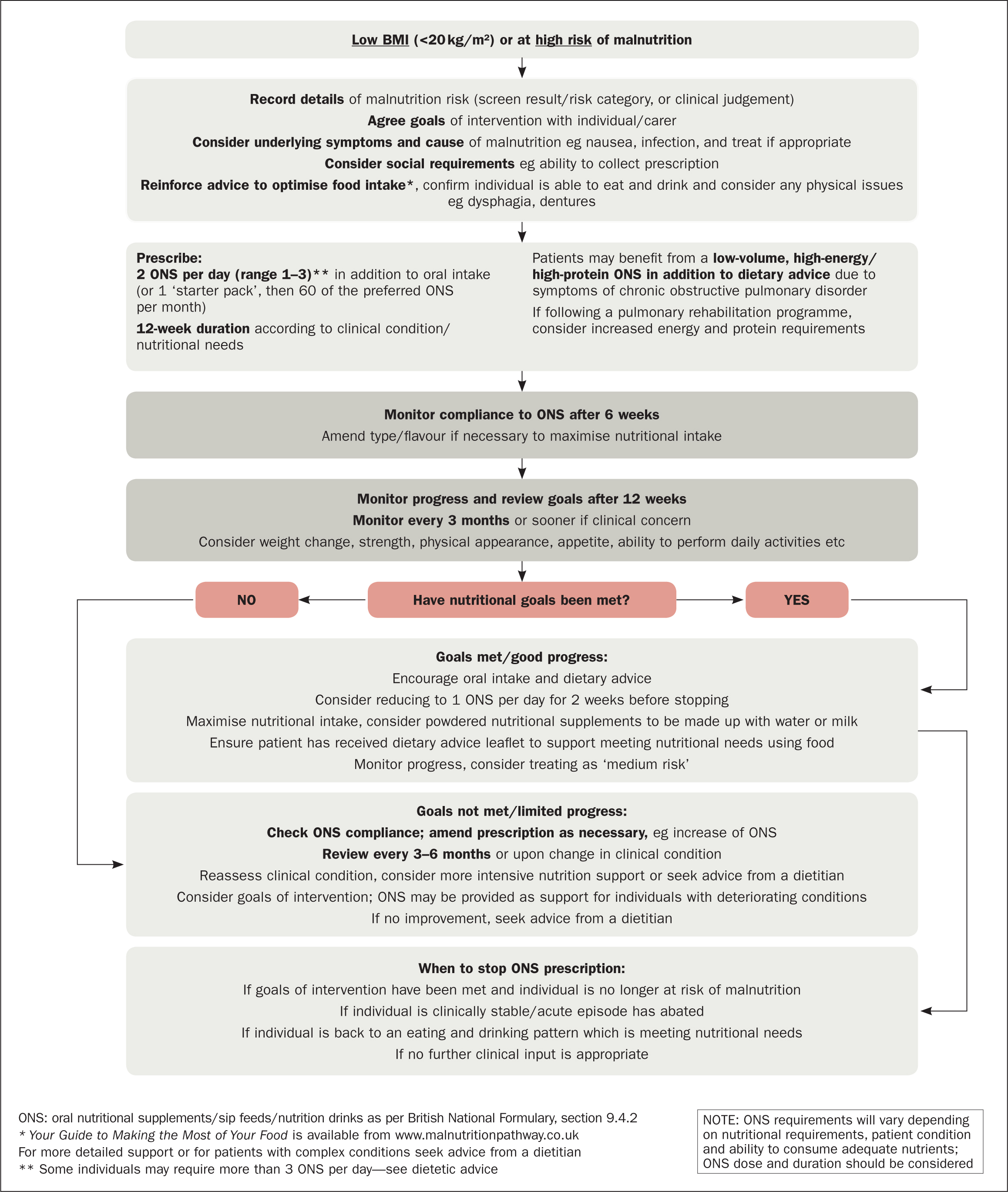
According to NHS England (2015), malnutrition is still poorly recognised in UK community and healthcare settings, despite numerous reports highlighting that individuals in these settings often receive inadequate nutrition and hydration (Francis, 2013). This means that local CCG guidelines may not reflect evidenced-based guidance on malnutrition management, including the prescribing of ONS, which may place patients at risk of increased severity of malnutrition and poor clinical outcomes (Johnson and Sykes, 2017).
In addition to this, financial pressures within the NHS mean that waiting times to seek therapeutic interventions, including nutrition and dietetic services, can in some areas be up to 8–14 weeks from the point of referral (NHS England, 2017). Such waiting times are at odds with the Managing Malnutrition in COPD guidelines, which stipulate that food fortification should be given as a first-line treatment to any patient with malnutrition to assist in weight loss prevention (Johnson and Sykes, 2017), and thus prevent an increased risk of malnutrition and further exacerbation of COPD and/or hospital admission (Hodson, 2017). This recommendation for early intervention is supported by NICE (2012; 2018), which outlines findings that demonstrate that there is a substantial cost benefit the quicker patients are identified as being at risk of malnutrition and offered treatment (NICE, 2012).
Prevention is better than cure
For people with lung conditions such as COPD, a nutritionally balanced, varied diet is particularly important to maintain a healthy body weight and prevent malnutrition (BLF, 2017b). A well-balanced diet includes five key food groups: fruit and vegetables; carbohydrates; protein; milk and dairy products; and fats (BLF, 2017b). Protein is essential in helping to maintain muscle strength (including chest muscles involved in breathing) and is also important for the immune system (BLF, 2017b). Ways of gaining weight include eating little and often, opting for higher calorie food options (such as full-fat milk and yoghurts) and stimulating the appetite by exercising or getting out into the fresh air (BLF, 2017b).
There is a growing body of evidence that ONS prescribed for those with COPD who are malnourished or at risk of malnutrition significantly improve exercise tolerance, dyspnoea, general wellbeing and quality of life (Collins et al, 2012; 2013; Ferreira et al, 2012). The Managing Malnutrition in COPD guidelines recommend that low-volume, high-protein, high-energy ONS are the best form of nutritional management, particularly for those whose predominant feature of COPD is increasing dyspnoea leading to high energy expenditure (Banner et al, 2016; Bostock-Cox, 2017).
The importance of preventing malnutrition in COPD is illustrated by the results of a pilot study that was undertaken to examine the effects of implementing Managing Malnutrition in COPD guidelines in the care of COPD patients in the community at high risk of developing malnutrition (Kominek et al, 2017). The study showed that prescribing Fortisip® (Nutricia) compact protein supplement to 19 malnourished COPD patients for 12 weeks resulted in improvements in weight, height, BMI and MUST scores. After 12 weeks, there was a substantial reduction in overall malnutrition risk in the patient group (based on MUST risk category), with the number of patients at high risk of malnutrition falling from 19 to 10. Overall, 53% of patients met the primary nutritional goal (weight maintenance over 12 weeks) and 47% met the secondary nutritional goal (weight gain over 12 weeks). The total cost savings were £80.82 per patient, equal to savings of £1535.58 over the 12 weeks of the study (Kominek et al, 2017).
Furthermore, in a budget impact analysis of the effects of implementing the NICE (2006; 2012) clinical guidelines and quality standard on nutritional support in adults, treating 85% of patients with malnutrition and COPD was shown to save £119 000–£154 0 00 per 100 000 UK general population (Elia, 2015). These findings are supported by a 1-year, prospective, observational pilot study conducted in 834 outpatients with COPD attending a large tertiary Australian hospital during 2011 (Hoong et al, 2017). The study showed that malnourished patients had a significantly higher 1-year mortality rate than non-malnourished patients (27.7% versus 12.1%; P=0.001) (Hoong et al, 2017). Malnourished patients were also hospitalised more frequently than those who were not malnourished and the length of hospital stay for malnourished patients was almost double that of non-malnourished patients (11.6 versus 6.7 days; P=0.003) (Hoong et al, 2017). Overall, the cost of treating malnourished patients was approximately twice that of treating patients who were not malnourished ($23 652 AUD versus $12 362 AUD; P=0.002) (Hoong et al, 2017).
Despite evidence demonstrating the benefits of early treatment of malnutrition in COPD (in terms of both patient outcomes and health economics), the prescribing of ONS is still often restricted on the basis of cost, since the Advisory Committee on Borderline Substances (ACBS) considers ONS to be a ‘borderline’ foodstuff; PrescQIPP advises that ONS should be prescribed only in exceptional circumstances (PrescQIPP, 2017). Guidelines from the Department of Health and Social Care (2019) suggest that these exceptional circumstances include ‘disease-related malnutrition’. However, this is generic guidance and there is an unmet need for specific guidelines for prescribing ONS for those with COPD.
The type of ONS used is also an important consideration. Some products require specialist preparation (eg whisking with milk), which may make compliance difficult and/or increase breathlessness from the effort required in preparation (Barker, 2011; BAPEN, 2018). Such issues can be addressed using ready-prepared ONS (Bostock-Cox, 2017). Moreover, compliance may be further improved with the use of small-volume, high-protein, high-energy ONS (typically 125 ml and >2 kcal/ml), which contain similar nutritional content to the higher-volume ONS that are often prescribed (250–300 ml and approximately 300 kcal/bottle), particularly in patients who are unable to consume large volumes (Hubbard et al, 2010; Hubbard et al, 2012; Hodson and Blamires, 2015).
Conclusion
Malnutrition is common in patients with COPD and can contribute to morbidity and mortality. However, it may be reversible and should not be seen as an inevitable part of the disease process.
Despite this, malnutrition is under-recognised and often untreated. Although guidelines for the management of malnutrition in COPD recommend first-line nutritional support, this recommendation is often not reflected in local guidelines or implemented at a local level.
The use of low-volume, high-protein, high-energy ONS may provide a pragmatic, cost-effective means of providing nutritional support to COPD patients who are malnourished or at risk of malnutrition, thereby helping to improve their physical health, general wellbeing and quality of life.
KEY POINTS
- Malnutrition is common in patients with chronic obstructive pulmonary disease (COPD) and associated with poor outcomes in terms of mortality, morbidity and quality of life, and increased healthcare costs
- Malnutrition in COPD is not inevitable and can be prevented or reversed
- COPD guidelines recommend screening for malnutrition and first-line management with nutritional support for people who are malnourished
- Patients with a BMI of <20 kg/m2 or who are at high risk of malnutrition should be given oral nutritional supplements (ONS), to increase their total calorific intake and promote weight gain
- ONS are a pragmatic, cost-effective means of providing nutritional support to patients with COPD who are malnourished or at risk of malnutrition, helping to improve their physical health, general wellbeing and quality of life
CPD reflective questions
- What are the main consequences of malnutrition in chronic obstructive pulmonary disorder (COPD), in terms of patient health and healthcare use?
- What are the key causes of malnutrition in COPD?
- What are the current recommendations regarding screening and nutritional support in the management of malnutrition in COPD, and what role do oral nutritional supplements play in this?

