The COVID-19 pandemic presents extraordinary challenging times worldwide, in particular for patients and healthcare systems (Bouadma et al, 2020; Chen et al, 2020; Huang et al, 2020; Zhou et al, 2020; Zhu et al, 2020). The nature of the virus means it is highly contagious and spreads rapidly. Patients present with a range of symptoms including fever, dry cough, fatigue, loss of smell and taste, dyspnoea and myalgia (Li et al, 2020a; Wu and McGoogan, 2020; Xiao et al, 2020; Zhao et al, 2020). Many COVID-19 patients also present with acute respiratory complications requiring admissions to intensive care units (ICU). Prolonged ICU admissions secondary to COVID-19 respiratory complications have been major causes of morbidity and mortality, particularly in older adults (Bouadma et al, 2020; Chen et al, 2020; Huang et al, 2020; Zhu et al, 2020). The virus has also been shown to affect the gastrointestinal tract (GIT) (Cheung et al, 2020; Yeo et al, 2020; Xiao et al, 2020) and renal and neurological systems (Li et al, 2020b; Pei et al, 2020) It is well documented that prolonged ICU admissions are associated with a loss of muscle mass, reduced functional capacity and an increased risk of malnutrition, resulting in a reduced quality of life and increased morbidities (Singer et al, 2019).
Nutritional Needs
Research relating to the metabolic function of patients with COVID-19 is scarce. Until more research is published, general nutrition principles involving critical care patients should be the focus of nutrition provision (Kreymann et al, 2015).
It is widely accepted that severe respiratory infections increase catabolism. This, in addition to increased energy expenditure associated with the mode of ventilation, increases energy and protein requirements. In conjunction with suboptimal nutritional provision, this results in calorie and protein deficiencies (Singer et al, 2019). The provision of early enteral nutrition (EN) in the general ICU setting improves mortality and reduces infections compared to when it is delayed or withheld (McClave et al, 2016; Taylor et al, 2016). It has been reported globally that ICU COVID-19 patients often have a reduced oral intake for 5–10 days prior to admission, have chronic diseases and are elderly (Fang et al, 2020; Huang et al, 2020). These are indications for initiation of early enteral feeding and aggressive nutritional support. International guidelines for nutrition during critical illness and COVID-19 ICU admissions recommends initiating early EN within 24-36 hours of admission to ICU or within 12 hours of intubation and placement on mechanical ventilation (Martindale et al, 2020). Additional International guidelines recommend enteral feeding should be commenced within 24-48 hours of admission to general ICU (Singer et al, 2019).
Enteral feeding is the preferred route and recent European guidelines suggest daily target intakes of 25kcal/kg and 1.3g protein/kg (Singer et al, 2019). Hypocaloric feeding not exceeding 70% of estimated energy requirements should be administered for the first week of the ICU admission, increasing to 100% after 7 days (Singer et al, 2019). For obese patients in the critically care setting, a daily target of 11-14kcal/kg of actual body weight and a range of 2-2.5g protein of ideal body weight is advised (Kreymann et al, 2015). Hypocaloric feeding is also recommended (Martindale et al, 2020). Jejunal EN can be considered if patients are vomiting or experience gastroparesis (Thibault et al, 2020). Parenteral nutrition should only be initiated if early EN or jejunal EN is not possible (Martindale et al, 2020).
Never Events
Nasogastric tube (NGT) feeding is commonly performed in adult patients generally without incident. Following reports of patient death and harm caused by misplaced NGTs, the National Patient Safety Agency (NPSA) issued alerts NPSA 2005 and NPSA/2011/PSA002 stipulating the use of pH indicator strips (specified to be CE marked in 2011) to confirm the correct position of the NGT. pH testing is used as the first-line test method.
Since the NPSA alert 2005, there has been a further 21 deaths and 79 cases of harm reported to the National Reporting and Learning System (NRLS). The main causal factor leading to harm from misplaced nasogastric tubes was misinterpretation of X-rays.
Between 2011 and 2016, there were 95 reported incidents describing fluid, medications or liquid feed being introduced into the respiratory tract, resulting in 32 patient deaths (NHS Improvement 2016). Of the 95:
In 2009, feeding into the lung from a missed placed nasogastric tube became a Never Event in England. Evidence from the Never Event reports suggests there are issues with X-ray interpretation at all times, and there may be increased risks from nasogastric placement or X-ray checking at night (NHS Improvement, 2016).
Table 1 shows the procedure for insertion of fine bore NGTs.
| Action | Rationale |
|---|---|
| Wash hands | To comply with hospital hand hygiene policy |
| Explain the procedure to the patient | Ensure that the patient understands the procedure |
| Arrange a signal (if patient is able to) by which the patient can communicate if the patient wants the health professional to stop (eg by raising his/her hand) | The patient is often less frightened if he/she feels able to have some control over the procedure |
| Assist the patient adopt a comfortable semi-recumbent position in the bed. Support the patient's head with a pillow. The head should not be tilted back | To allow for easy passage of the tube. The slightly flexed forward position enables easy swallowing and ensures the epiglottis is not obstructing the oesophagus, reducing the risk of tracheal intubation |
| Select the required length of NG tube by measuring the distance from the tip of the nose, to the earlobe and to the bottom of the xiphisternum (the NEX measurement) | To ensure the appropriate length of tube is passed into the stomach |
| Lubricate the tip of the tube with sterile water | To facilitate passage of the tube |
| Check the patient's nostrils | To determine if they have polyps or a deviated septum. If they do, not to pass NGT |
|
Patients who can swallow
|
A swallowing action closes the epiglottis, enabling the tube to pass into the oesophagus |
| Advance the tube through the pharynx, as the patient swallows until the NEX measurement. If the patient shows signs of distress (eg gasping or cyanosis) remove the tube immediately | The tube may have accidentally been passed down the trachea instead of the pharynx. Distress may indicate that the tube is in the bronchus. Note: Do not interpret absence of respiratory distress as an indicator of correct positioning |
| If a guide-wire is kept in position, attach a syringe and aspirate a specimen of stomach contents using a 50ml enteral syringe | To obtain aspirate |
| Test aspirates and define the pH with CE marked pH indicator strips | To ensure the NGT is in the correct position with a pH 5.5 or below indicating it is safe to initiate feed |
| Once the tube is in the correct position secure the tube to the nostril with adherent dressing tape. Use hypoallergenic tape if required. An adhesive patch (if available) will secure the tube to the cheek | To hold the tube in place and reduce the risk of dislodgement and to ensure patient comfort |
| Flush the tube with 30ml of water | To remove any aspirate debris. The tube is now ready for feed and medications |
| Document the make and model of the tube as well as the date and time of insertion on the Adult Nasogastric Tube Insertion and Monitoring Chart | To maintain accurate records of the procedure |
Nasogastric Tube
NGTs are long polyurethane or silicone tubes that are passed through the nasal passages via the oesophagus into the stomach. They are passed ‘blind’ as a bedside procedure by registered nurses and doctors and trained MDT members. This means that the tube passes out of sight through the nose and throat, but anatomical structures direct it towards the GIT. Fine-bore feeding tubes should be used wherever possible as these are more comfortable for the patient and are less likely to cause oesophageal irritation and gastritis (Payne-James et al, 2001).
Nasojejunal Tube
Nasojejunal tubes (NJTs) feed directly into the small bowel and are indicated in delayed gastric emptying, persistent vomiting, severe gastro-oesophageal reflux or as a way of overcoming large gastric residual volumes (McClave et al, 2016).
They can be placed endoscospically by the gastroenterology team in endoscopy or on ICU. Alternatively, they can be passed at the bedside using the Cortrak 2 system that is discussed later in the article. If a dual lumen tube is not in-situ, then a separate NGT is required for gastric aspirates and administration of medications (McClave et al, 2016).
Clinical Audit Data
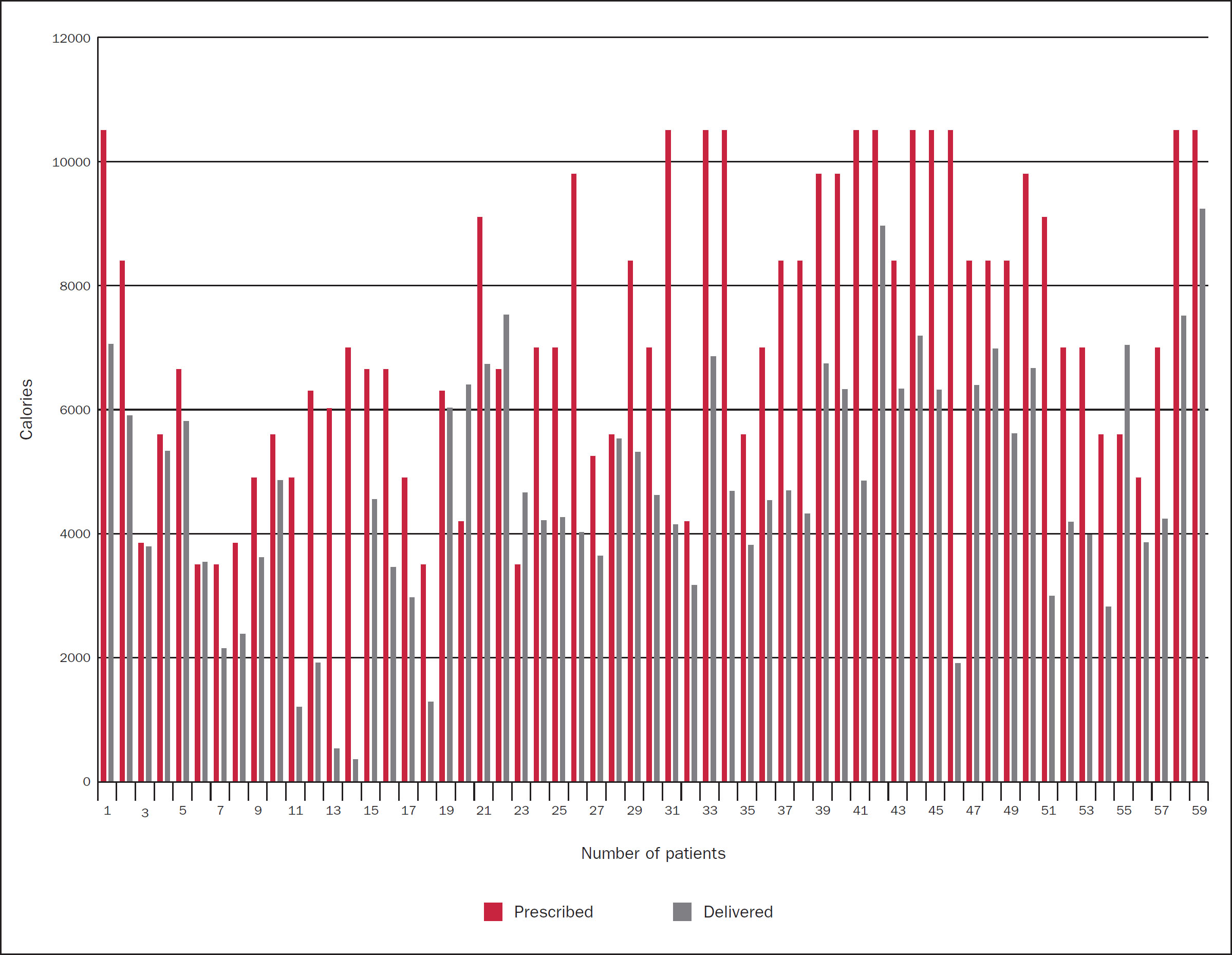
Using subjective global assessment, it was noted by the ICU team that many of the COVID-19 patients looked like they had experienced a large amount of weight loss during their hospital stay. This lead the ICU dietitian to retrospectively audit delivered versus prescribed feed for the 7 days of admissions for all ventilated admissions to the unit.
Fifty-nine admissions were included in the data analysis and all had an NGT inserted within 12 hours of intubation as per recommended enteral feeding ICU guidelines (Martindale et al, 2020). In the early stages of the pandemic (March and April 2020), the Cortrak 2 was used for NGT insertion on ICU admissions and NGTs were placed blindly for all admissions intubated in A&E. Enteral feed was commenced on safe confirmation of NGT placement.
Figure 1 illustrates that only 10% of admissions achieved or exceeded their prescribed volume of feed during the first 7 days of admission, with 6% of admissions being within 10% of achieving daily prescribed targets. Table 2 highlights the breakdown of admissions that received a percentage of their prescribed feed. The top three reasons for such poor feed delivery were proning, high gastric residual volumes and the nature of the pandemic. Patients were not fed proned in the initial phases due to increased risk of aspiration, which could compromise patients respiratory function. High gastric residual volumes increase the risk as aspiration in patients which again, could compromise patients respiratory function. It is important to remember the unprecedented pressure that healthcare systems were under at this time. At the peak of the pandemic, Chelsea and Westminster Foundation Trust's capacity had tripled in 3 weeks. The ratio of ICU nurses to patients was one to four and they were overseeing non-ICU trained staff who were looking after ICU patients.
| Percentage | 10 | 25 | 50 | 75 |
| Admissions | 4 | 9 | 29 | 12 |
The suboptimal delivery of feed resulted in an average weekly deficit of 4 050kcal and 229g protein per admission.
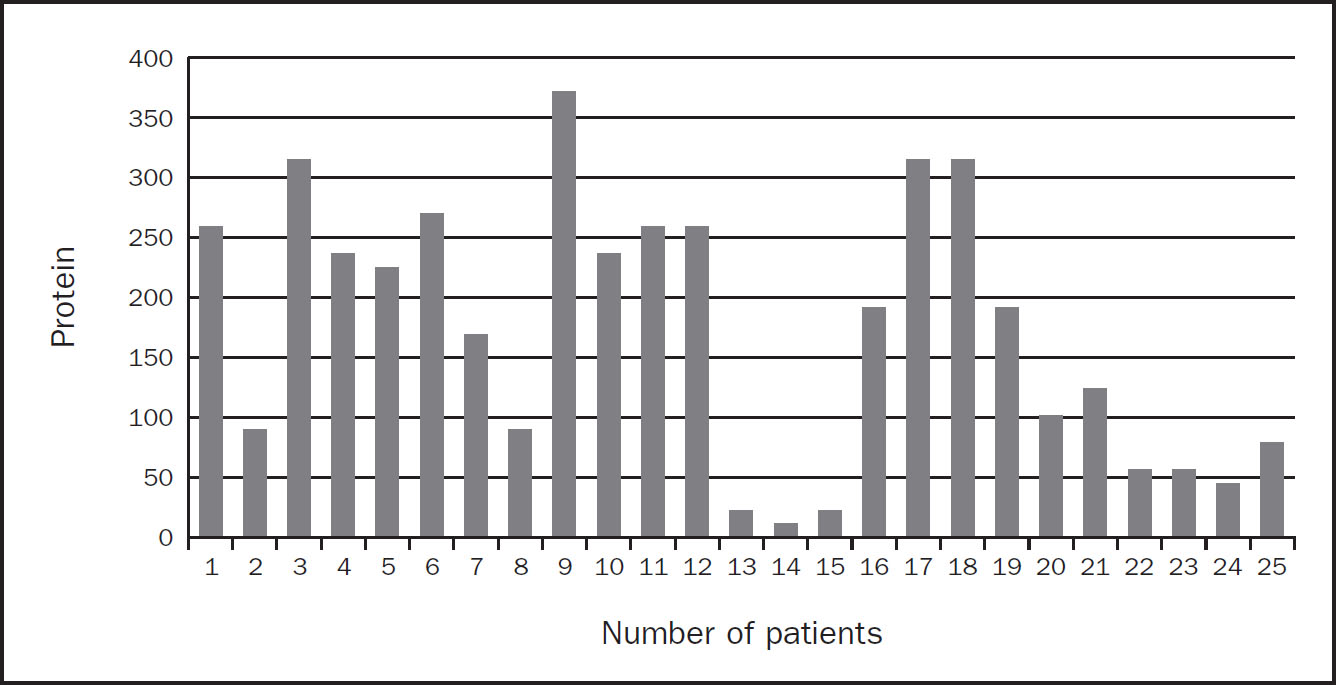
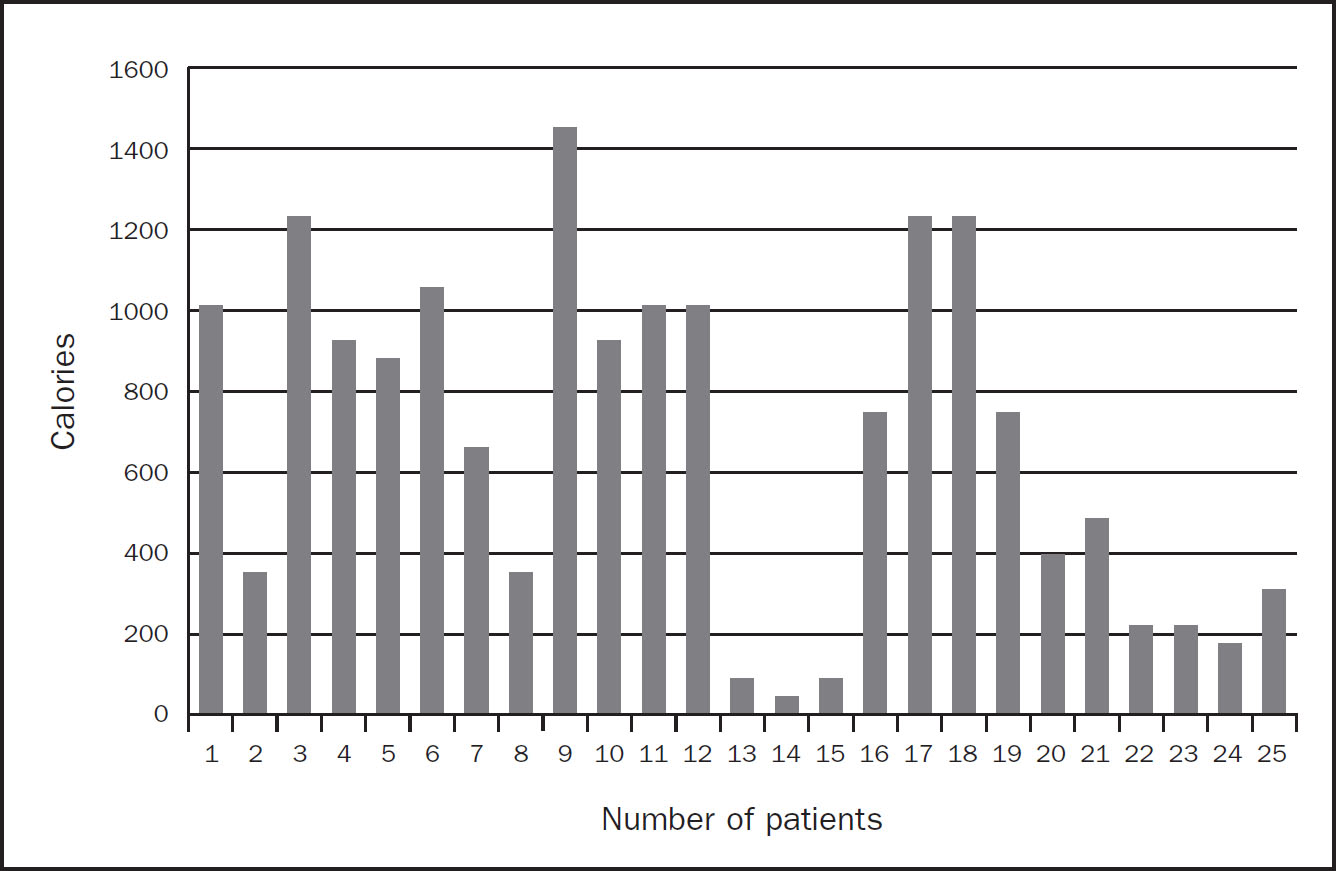
There were 25 admissions who were prescribed additional protein supplements in order to meet estimated requirements. Unfortunately, delivery of these protein sachets was suboptimal resulting in a further protein and calorie deficit as per Figure 2 and 3, respectively. The top three reasons for this were also due to proning patients, high gastric residual volumes and the nature of the pandemic. As a result the average weekly calorie and protein deficits for these 25 patients were 689kcal and 169g protein per admission.
Cortrak
The Cortrak Enteral Access System (ENS) uses electromagnetic sensing technology to track live placements of NG and NJTs at the patient's bedside. It is designed to safely guide the placement of feeding tubes and to help confirm they are placed correctly. It provides the trained user with immediate visualisation for interpretation that feeding tubes are in the correct position and can be used immediately (with pH 1–5.5 for NGTs). This reduces delays in waiting for X-ray confirmation and optimises immediate feed delivery for patients.
An electromagnetic sensor tracks the path of the feeding tube during the procedure. The system consists of a monitor unit, a single use polyurethane radiopaque tube, inclusive of a electromagnetic tipped stylet and a receiver unit. The receiver unit tracks the electromagnetic signal from the transmitting stylet to the monitor unit. Once the patient is correctly positioned, the receiver unit is placed over their xiphisternum and the stylet is connected to the monitor using the interconnecting cable. The monitor then displays real-time representation of the feeding tube using anterior, lateral and depth cross-section views. It is important to note, correct placement of the receiver unit is essential in order to gain accurate results.
Chelsea and Westminster Hospital Foundation Trust introduced this ENS in 2013. The catalyst was driven by delayed endoscopic tube placements for burns ICU patients. Late endoscopic NJT placements for the BICU cohort resulted in poor feed delivery and patients not meeting their daily prescribed volume of feed as they were kept nil by mouth for theatre and large dressing changes. It was also noted by the ICU and Burns dietitian, that there would be a significant improvement in patient care coupled a significant financial saving for the Trust as endoscopic NJT and parenteral nutrition costs could be reduced. The Cortrak ENS was used predominately for NJT placements in BICU and ICU patients by the ICU dietitian. In 2016, a successful business case was submitted and training and successful insertion of NG and NJTs for all ICU and BICU patients commenced for registered ICU nursing staff.
The company Cortrak training package was straightforward and easily accessible online. It consisted of pre-course training, followed by face-to-face classroom training and supervision of tube insertion with patients. Once the user completed a minimum of three accurate insertions, they were deemed competent to use the equipment and issued with account login details.
The introduction of the Cortrak ENS to Chelsea and Westminster Hospital ICU and BICU has proved invaluable as it has improved patient care and the provision of enteral feeding, reduced delayed feeding times and has reduced cost of parenteral nutrition for the Trust. All registered nursing staff, consultants and the ICU dietitian are trained to insert NGTs and the ENS is used to re-check NGT position should there be any query of tube displacement. If the trained user is unsure of a successful placement and is unable to obtain an accurate pH, a senior member of staff is approached or a chest X-ray is requested.
Case Study 1
A 62-year-old patient was admitted to the ICU at Chelsea and Westminster Healthcare NHS Foundation Trust during the pandemic with a 10-day history of shortness of breath, largely bedbound for a week prior to admission, reduced oral intake and shortness of breath going to the toilet. Subsequently, the patient was diagnosed as COVID-19 positive.
The patient was fit and well prior to admission, lived alone, reported a good exercise tolerance and no recent travel.
A Cortrak 2 NGT was inserted after intubation and enteral feed was commenced as per ICU protocol. After 3 days in the ICU, it was necessary to prone the patient for respiratory management. As this was in the very early stages of the pandemic (March to April 2020), the decision was made to hold enteral feed. Once deproned, enteral feed was restarted. This pattern occurred for 4 days and the patient did not receive feed for 16 hours whilst proned. The decision was made to place a Cortrak 2 NJT to feed the patient and minimise aspiration risk.
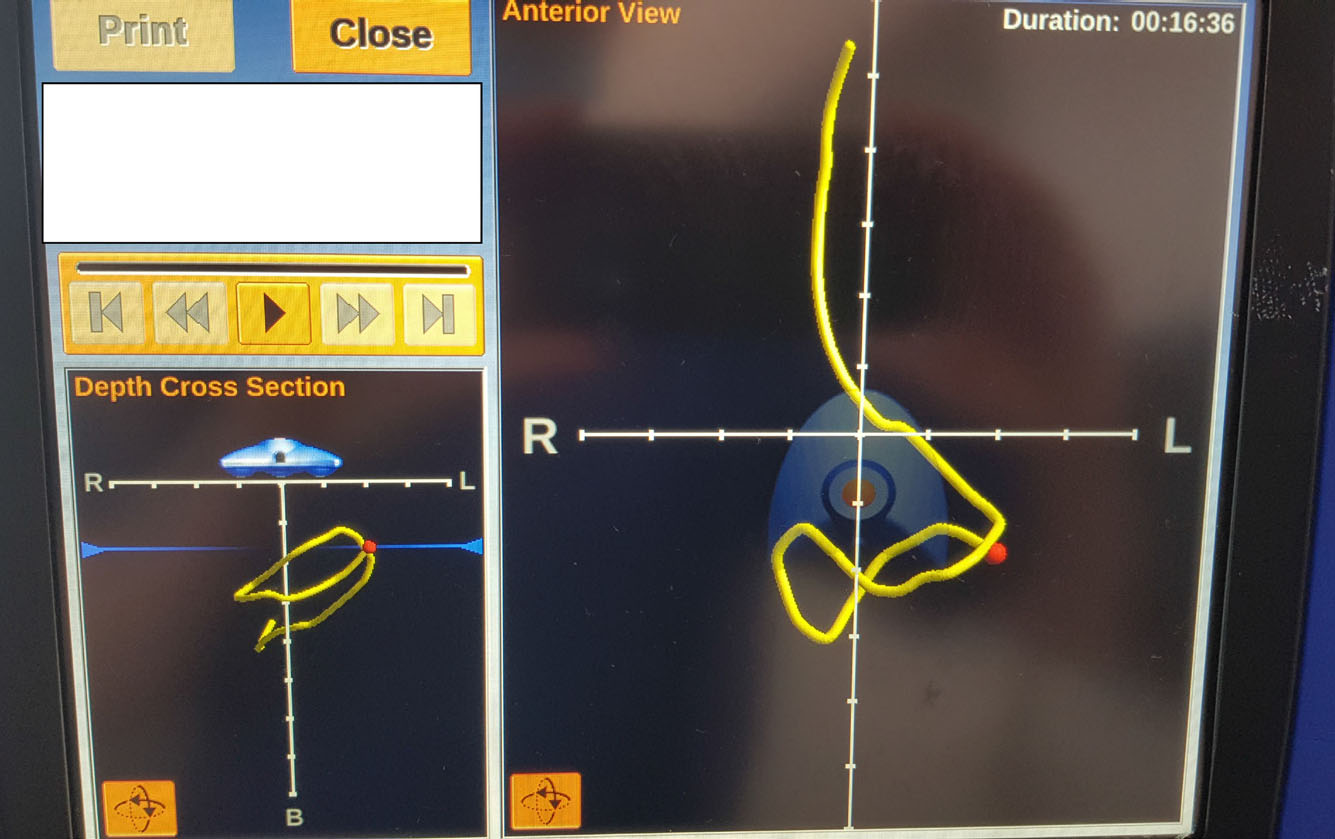
On day 5 of admission, an NJT was inserted (Figure 4). It was a challenging placement as the patient was extremely unstable medically and desaturated when repositioned. This required the ICU registrar to be present during the placement to monitor blood pressure. Once the NJT was in situ, it was secured using a feeding tube retention system, as further proning periods were predicted. Feed was commenced after NJT placement and daily prescribed target volume of feed was achieved.
Case Study 2
A 34-year old patient was admitted to the ICU at Chelsea and Westminster Healthcare NHS Foundation Trust during the pandemic with a 5 day history of history of fever, increasing shortness of breath and a 2 day history of haemoptysis. The patient was brought in by ambulance to A&E, intubated and transferred to ICU. Subsequently, the patient was diagnosed as COVID-19 positive.
The patient was morbidly obese weighing 207kg and a BMI of 62kg/m2 and had recently travelled abroad and became unwell after returning to the UK.
A Cortrak 2 NGT was inserted when the patient arrived to the ICU. This admission was at the peak of the pandemic and patients were located in theatres, recovery and ICU. Despite being an NGT placement, which should have been straightforward, the patient was sitting up on a bariatric bed, intubated and sedated. Due to the level of obesity, the xiphisternum could not be located. Correct placement of the receiver unit is essential in placement. Due to the patient's position in bed, the operator required two staff members to hold the weighted stabiliser in position to ensure the receiver unit remained in position. Despite this, the operator placed the NGT correctly and confirmed position with a correct pH. Enteral feed was commenced immediately and there was no need for a chest X-ray to confirm position, as requested by the ICU team.
Case Study 3
An 86-year-old patient was transferred to the ICU at Chelsea and Westminster Healthcare NHS Foundation Trust from an ICU during the pandemic with a 9 day history of history of cough, shortness of breath and tachycardia. The patient was diagnosed as COVID-19 positive and arrived to the unit intubated and sedated with an ENFit compliant Ryles NGT in situ.
The patient presented with ischaemic heart disease— percutaneous coronary intervention with stent insertion 2019, was on dual antiplatelet therapy, hypercholesterolaemia and angina. Despite this, the patient was fully independent of all daily activities and continued to work in the family business. They were an ex-smoker, stopping in the 1970s, enjoyed alcohol occasionally and lived with their partner.
Enteral feeding commenced via an ENFit compliant Ryles tube; however, it became very apparent this was not tolerated as the patient vomited. The vomiting continued despite using motility agents and a reduction in feed rate. The ICU team requested a Cortrak 2 NJT which was placed the following day (Figure 5).
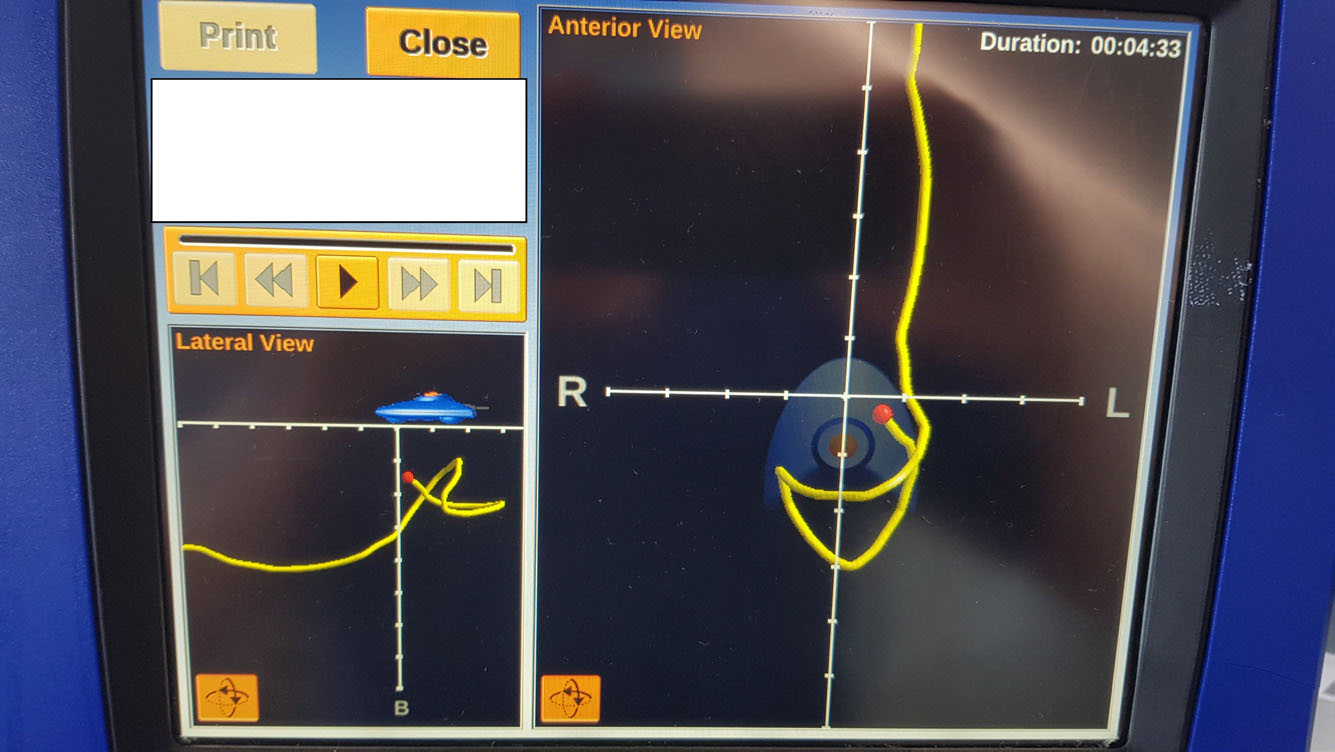
Placement was challenging due to large gastric volumes which were aspirated using the ENFit NGT. The feeding tube was difficult to pass at the point of the pylorus; however, repositioning the patient so the lay on their right side, helped with the successful placement. Feed was commenced immediately and no further vomiting occurred.
Conclusion
The COVID-19 pandemic has challenged and continues to challenge healthcare systems, clinical staff, health care pathways, patients and families globally, in a manner that could never have been imagined before. Healthcare systems have responded as best as they could in the traumatic times they faced. As a team, we reflected on clinical practice during this time and delivery of enteral feed was one area of focus. Audit results clearly showed delivery of enteral feed was poor and unacceptable and this allowed us to identify areas to improve upon should there be a second wave:

