MDRPUs continue to present a considerable problem (Young, 2018). In the year up to September 2017, 50 402 incidents relating to the use of medical devices were reported to the National Reporting and Learning System, with harm being caused in 7230 of these cases. Any medical device can cause pressure injury in any patient (Byrant, 2012); however, the risk is particularly high for patients at the end of life (Chaplin, 2000; Sibbald et al, 2009) and elevated in patients with cancer (Hanonu and Karadag, 2016) or patients undergoing surgery or in intensive care with a history of cancer, cardiovascular disease, peripheral vascular disease, pulmonary disease, neurological disease and/or diabetes mellitus (Hanonu and Karadag, 2016; Delmore and Ayello, 2017; Amirah et al, 2017).
In 2018, MDRPUs were added to the NHS Improvement framework for the measurement and definition of pressure ulceration (NHS Improvement, 2018), which states that this injury should be reported separately. There is little or no published evidence on the costs associated with MDRPU, particularly the substantive indirect costs associated with litigation and insurance (Gefen et al, 2020).
MDRPUs can cause additional morbidity and reduce quality of life (Gefen et al, 2020). In 2020, Journal of Wound Care published an expert opinion consensus document on the prevention of this form of injury, which it termed device-related pressure ulcers (DRPUs), in recognition that all devices, not just medical ones, can cause harm (Gefen et al, 2020). The consensus document defined DRPU as:
‘A DRPU involves interaction with a device or object that is in direct or indirect contact with skin, or implanted under the skin, causing focal and localised forces that deform the localised and deep underlying tissues. A DRPU, which is caused by a device or object, is distinct from a PU, which is caused primarily by body weight forces. The localised nature of device forces results in the appearance of skin and deeper tissue damage that mimics that of the device in shape and distribution.’
Pressure ulcers (PUs) occurring on the nasal bridge, nares (nostrils), fingertips, ears and lips can only be attributable to the devices located in this area (Black et al, 2010), although lack of assessment and care can also be involved (Young, 2018). MDRPUs not only cause patient suffering, with potential for disfigurement and loss of function, but also are costly. They are generally associated with hospital settings and involve tubing, such as nasogastric, oxygen and endotracheal, and ventilation masks, all of which are medical devices. A prevalence study found that the medical device most associated with MDRPUs was nasal oxygen tubing (32%), which affects both the ears and the nose (Kayser et al, 2018).
Prevention
It is much more difficult to prevent MDRPUs than standard PUs (Fletcher, 2012). Use of medical devices is unavoidable in some patients, and it is not always possible to examine the area under the device, such as when adjusting or relocating a therapeutic device is contraindicated, or when use of a life-sustaining vascular device precludes patient turning or repositioning, or when underlying oedema is present (Young, 2018).
Many institutions have reduced the incidence of traditional PUs through quality improvement initiatives (Black et al, 2015); in so doing, MDRPUs may have become more apparent, leading to the current focus on them. Prevention of PUs or MDRPUs requires a high level of awareness and rigorous adherence to practices that minimise the risks (Gefen et al, 2020), principally skin care and maintenance of skin integrity.
Variations in the quality and availability of NHS care have necessitated the development of a culture where patient safety is everyone's concern (NHS England, 2014). Any MDRPU strategy should involve both the patient and their family and carers (Mitchell, 2018). In this way, health professionals can reduce the risk of skin breakdown. Prevention strategies could include higher levels of vigilance, involving nurses in product selection and quality improvement initiatives (Haugen, 2015). Other elements could comprise more efficient strategies for reporting MDRPU, recognising which devices pose a higher risk, informing manufacturers when their devices are observed to cause harm, and lobbying for device redesign to enhance patient safety. To minimise risks, attention must be paid to device placement, securement, positioning on the skin, forms of protection and regular skin assessment (Schofield, 2020).
The SSKIN care bundle, which has modules on surface, skin, keep moving, incontinence, nutrition, is a nationally recognised tool that can be used to define best-practice interventions to aid the elimination of facility-acquired pressure damage (Department of Health, 2011). In 2018, NHS Improvement (2018) introduced the aSSKINg model, which includes two additional modules, assessing the risk and giving information, that underpin and support successful implementation of care.
Commonly documented prevention measures include repositioning medical devices at regular intervals, adding protective layers between the skin and the device, securing devices with appropriate tapes to stop them moving and causing friction, monitoring the level of moisture on the skin (Black et al, 2015; Gefen et al, 2020; Latimer et al, 2017) and using specific pressure-relieving dressings when required (Schofield, 2020).
Gefen et al (2020) developed the SECURE mnemonic for an integrated pathway for DRPU prevention, which can be used in addition to the aSSKINg bundle (Table 1).
| S | Skin/tissue | Thorough assessment should be undertaken daily or more frequently, depending on risk Handover to ensure continuity of care |
| E | Education | Health professionals, patient, carers, family and industry |
| C | Champion/collaborate | Lead the adoption of evidence–based devices developed through collaboration with the manufacturer and health professionals |
| U | Understanding | Develop a thorough understanding of the causes of device-related pressure ulcer (DPRU), patient assessment and correct product use |
| R | Report | Ensure DRPU is correctly reported in a timely manner |
| E | Evaluate | Evaluate devices for their ability to minimise DRPU by thoroughly analysing support data and conducting clinical evaluations |
Source: Gefen et al, 2020
Risk factors for MDRPU
An understanding of the risk factors for MDRPU can improve prevention. The longer the device is used, the higher the risk of MDRPU occurrence (Gefen et al, 2020). Many patients rely on medical devices to live (Chen, 2018). Such devices include continuous positive airway pressure (CPAP) masks, oxygen therapy tubing and endotracheal tubes, as well as less critical devices such as orthotic devices, indwelling lines and bedframes (Gefen et al, 2020).
The exact cause of the ensuing ulceration has not been elucidated, but the logical assumption is that when a device is tightly secured to the patient's body, it can apply increased pressure to the tissues and cause oedema (Black et al, 2015). Oedema under the device stretches the skin, making it more fragile and prone to pressure ulceration.
Another risk factor for MDRPU is the tightness of securement method (Baldwin, 2002). The presence of moisture on the skin under the medical device, due to diaphoresis or secretions, can cause maceration, making it susceptible to pressure ulceration (Black et al, 2015). In cases of local oedema under the medical device, the risk of MDRPU will increase if the following comorbidities are present:
Subjecting skin and underlying soft tissues to external loads can reduce tolerance to pressure and shear forces and increase the likelihood of injury (Bader et al, 2019).
All devices selected for application onto a patient should be assessed for their risk of causing MDRPU. In addition, patient factors that increase the risk of MDRPU, such as incontinence, malnutrition and altered levels of consciousness or sensation, should be identified in care plans (Schofield, 2020).
Predictive risk factors in the oncology setting are illustrated in Table 2.
|
|
Source: Maskai et al, 2007
Role of soft silicone dressings in preventing and treating MDRPU
Foam dressings appear able both to absorb moisture and reduce pressure (Black et al, 2015). Huang et al (2009) showed that use of silicone foam dressings as a lining during nasal intubation reduced the size and severity of nasal-alar PUs. This encouraged the author to evaluate the effectiveness of silicone dressings as a prophylaxis when placed under a medical device on a vulnerable patient's skin.
Soft silicone dressings are a family of solid, but soft and tacky, medical devices. They were developed to minimise pain and trauma at dressing change and to protect the peri-wound skin (Lawton and Langeon, 2009). As such, they do not cause trauma to the wound or peri-wound skin during removal (Majan, 2006).
Matsumura et al (2014) found, in a comparative study, that dressings containing a silicone adhesive removed less stratum corneum from the wound when compared with adhesive hydrocolloid and polyurethane foam dressings. Timmons et al (2009) found that use of silicone dressings not only improved patients' quality of life by reducing pain on removal and thus anxiety, but also accelerated the healing process. A study by Santamaria et al (2015) concluded that the use of a multi-layered silicone dressing reduced PU incidence in intensive care units when used on the sacrum and heels.
Nurses are expected to provide high-quality, evidence-based care that is also cost effective (Drewery, 2015). This small product evaluation did not focus on wound healing, but rather on the ability of Kliniderm foam silicone lite to prevent the occurrence of PUs under medical device placed over the ears and nose. All patients consented to take part in the case studies.
Case study 1
A 61-year-old lady was admitted with tumour progression of the left lung with a moderate pleural effusion. She was undergoing chemotherapy and immunotherapy, and was on steroids, all of which contributed to poor skin integrity.
The patient was receiving continuous oxygen therapy through a nasal cannula. As part of an aSSKINg bundle prevention strategy, Kliniderm foam silicone lite was applied under the oxygen tubing on her ears to prevent pressure ulceration.
Risk assessment identified that she had a Braden score of 20, placing her at high risk of pressure ulceration (Bergstrom et al, 1987). However, her lifelong need for oxygen, due to the disease progression and breathlessness, meant she was also at high risk of MDRPU.
The Braden scale does not include external risks for pressure injury, including medical devices (Valeri and Struffenegger, 2019). In addition, optimal cut-off scores have not been developed for each patient population (eg oncology) or care setting. Therefore, nurses need to use their clinical judgment when assessing risk as part of a PU prevention strategy (National Institute for Health and Care Excellence, 2014).
This lady is independently mobile, with no other risk factors. Kliniderm foam silicone lite was wrapped around the oxygen tubing and rested on the ear, which she said felt comfortable (Figure 1).
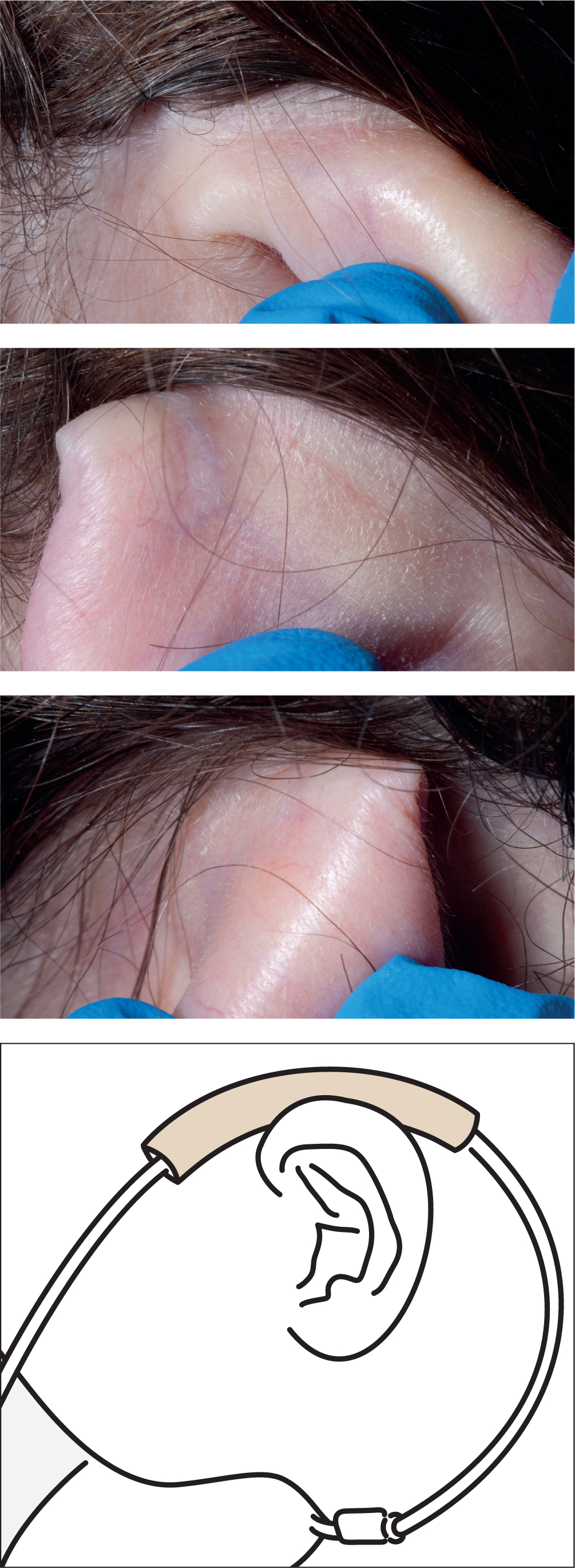
After 3 weeks of hospital admission to relieve symptoms of pleural effusion and breathlessness, she did not develop any erythema on her ears. She was discharged home, where this prevention strategy was maintained.
Case study 2
A 73-year-old man was admitted with metastatic non-small-cell lung carcinoma, with lung, bone and ureteric metastasis. While receiving end-of-life care, he developed a chest infection, which increased his oxygen requirement. Unfortunately, non-blanching erythema with minor skin breakdown occurred on his right ear and the nasal areas under the nasal oxygen cannula (Figure 2).
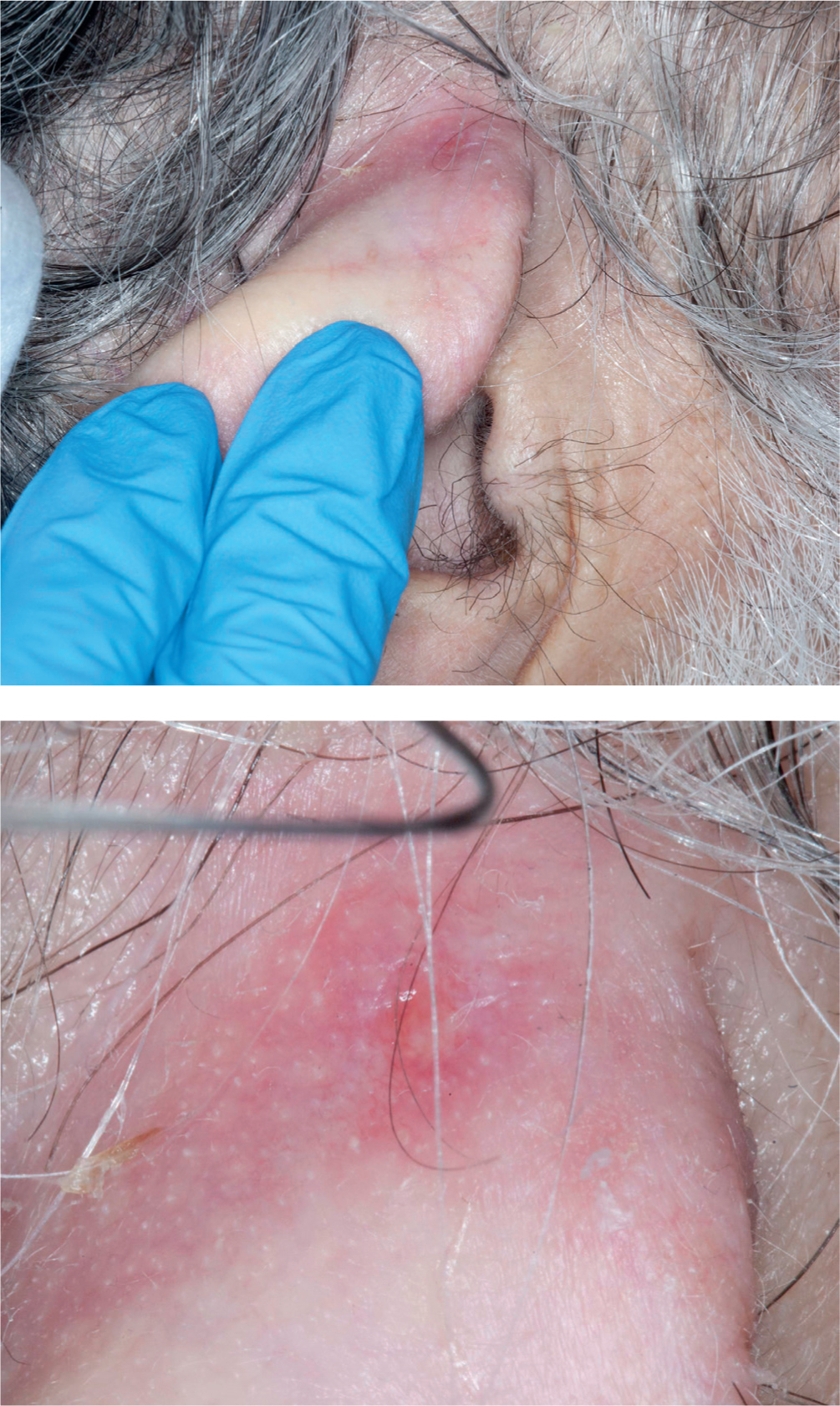
Kliniderm foam silicone lite was cut into strips and applied, as a prophylaxis, beneath the tubing on his ear and nasal area where the cannula was resting. As the dressing's silicone contact layer is adherent, the dressing stayed in place in both locations. Meanwhile, the areas in which the dressings were located were assessed every two hours and the medical devices repositioned. Within 5 days, the erythema had resolved and further skin breakdown was avoided.
Sadly, the patient died peacefully soon after. However, the care team had offered great support by managing his pain and avoiding further skin damage and MDRPU.
Case study 3
A 50-year-old lady was admitted with progressive metastatic breast cancer and symptomatic malignant recurrent pleural effusion. She was receiving active chemotherapy, but this had to be discontinued due to her worsening symptoms, and she was now receiving end-of-life care.
The patient required 5 litres of oxygen via a nasal cannula. Her management plan included frequent skin assessment and strategies to reduce risk of skin breakdown (Gefen et al, 2020). Kliniderm foam silicone lite was applied under the oxygen tubing to prevent MDRPU on her ear. It was cut into strips and used on both ears underneath the tubing. Due to its range and shape, it was possible to cut the dressing to size and apply it directly onto the skin (Figure 3). The silicone held the dressing in place with no need for any additional tape. The dressing was peeled off to observe the skin during the skin assessments, after which the devices were repositioned as per the care plan.
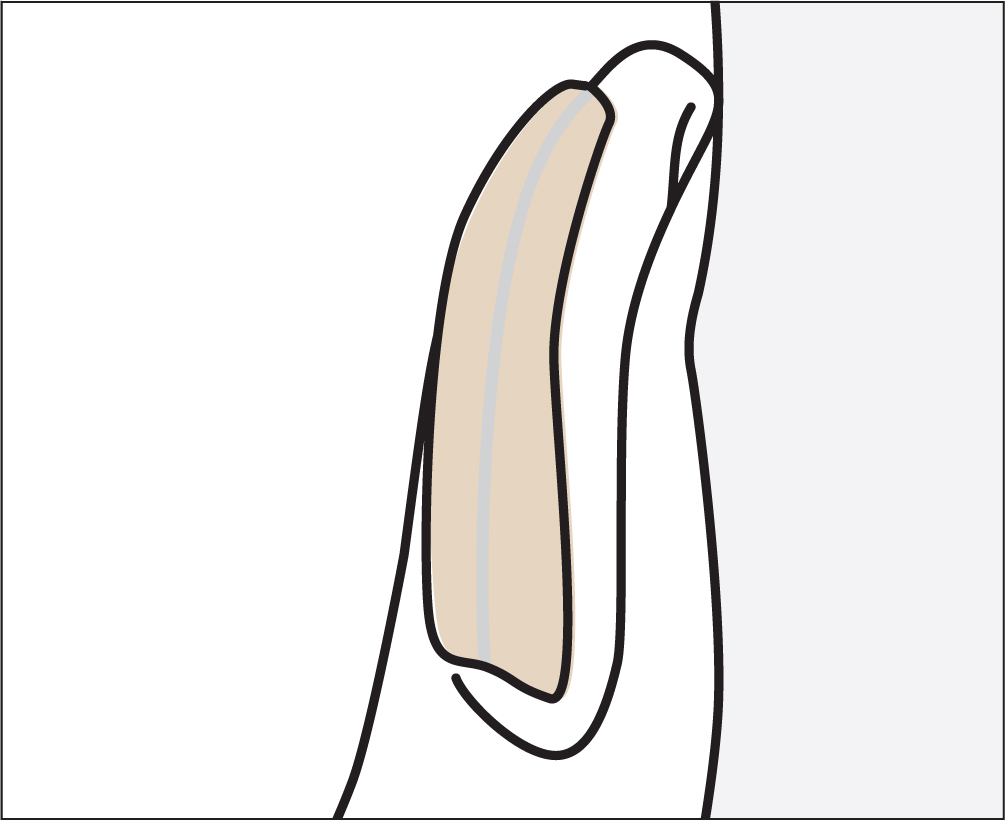
The patient was nursed on a high-specific dynamic mattress in hospital. She went home to spend her last few days with her family, with the same prevention strategy in place.
Conclusion
Use of medical devices is widespread in all clinical environments, but this is associated with an increased risk of MRPU. Indeed, it could be argued that this is inevitable in critical situations. Regular risk assessment and skin inspection should be undertaken to identify high-risk patients. In the author's oncology setting, the use of Klinderm foam silicone lite under the medical devices, in combination with other prevention strategies, proved to be a valuable addition to the care plan. The introduction of a simple clinical strategy to reduce MDRPU can ensure that equipment used as part of the treatment causes little or no harm to patients. The product evaluation continues to monitor the effectiveness of the Kliniderm foam silicone lite dressings in reducing the risk of MDRPUs. Since the time of writing, Kliniderm foam silicone lite has been used on a further eight patients from the same setting, none of whom developed an MDRPU.

