While 90% of breast cancers occur as a result of an accumulation of somatic genetic changes, approximately 5–10% are believed to originate from inherited germline genetic variations. These cancers are most likely to be pathogenic variants in tumour suppressor genes such as the breast cancer susceptibility genes BRCA1 and BRCA2 (Brody and Biesecker, 1998; Alberg et al, 1999; Eccles and Pichert, 2005; Zhang and Powell, 2005). BRCA1 was mapped on chromosome 17 in 1990 (Hall et al, 1990) and subsequently cloned in 1994 (Miki et al, 1994) and BRCA2 was mapped on chromosome 13 in 1995 (Wooster et al, 1995).
Pathogenic variants in other genes, including ATM, BARD1, BRIP1, CDH1, CHEK2, MRE11A, MSH6, NBN, PALB2, PMS2, PTEN, RAD50, RAD51C, STK11 and TP53 are now also known to increase the risk of breast cancer, but research continues into the penetrance and overall risks associated with these genetic variants (Wang et al, 2018).
Pathogenic BRCA1 and BRCA2 variants are most closely associated with an increased risk of breast and ovarian cancers, but recent evidence also documents they increase the risk of other cancers, including pancreatic cancer and prostate cancer (Mersch et al, 2015).
The global female population has an overall lifetime risk of 12.5% of developing breast cancer and 1.3% of ovarian cancer (Howlader et al, 2019), and lifetime risks of developing breast and ovarian cancers are much higher when a pathogenic BRCA1 or BRCA2 genetic variant has been inherited (van Egdom et al, 2020).
Penetrance estimates for pathogenic variant carriers have been found to vary substantially between studies, but recent research estimates the cumulative breast cancer risk to age 80 years as being 72% for women carrying the BRCA1 and 69% for those with BRCA2 pathogenic variants. The cumulative ovarian cancer risk to age 80 years has been reported as being 44% for BRCA1 and 17% for BRCA2 pathogenic variant carriers. BRCA-associated cancer risks have also been shown to differ by cancer family history and the location of the genetic variation on the gene (Antoniou et al, 2003; Chen et al, 2006; Kuchenbaecker et al, 2017).
In addition to having a higher lifetime risk of developing breast cancer, women carrying a BRCA pathogenic variant also have a higher incidence of triple-negative breast cancer (TNBC) (Greenup et al, 2013). TNBC is a term that reflects a lack of immunostaining for receptors to the hormones oestrogen and progesterone and the protein HER2 and, because of this, treatment options are usually more limited and less targeted (Bianchini et al, 2016; Collignon et al, 2016). It is estimated that 15–20% of all breast cancers are classified as TNBCs and a higher incidence of TNBC is seen in women with BRCA-associated cancers, particularly BRCA1, and those who are diagnosed with a TNBC under the age of 50 years have at least a 10% chance of carrying a pathogenic BRCA variant, with the majority (80%) of these being BRCA1 (Evans et al, 2011).
BRCA genetic testing
In the UK, the current National Institute of Health and Care Excellence guideline for treatment of people with a family history of breast or ovarian cancer recommends that a woman with a 10% or more likelihood of being a pathogenic BRCA variant carrier should be offered genetic testing (NICE, 2019). This likelihood is usually calculated based on a person's family history of breast and other cancers using criteria such as the Manchester Scoring System or BOADICEA score (MacInnis et al, 2013; Evans et al, 2017).
Increasing evidence suggests that basing testing on family history alone may miss a significant number of women carrying the pathogenic BRCA variant (Robertson et al, 2012; Grindedal et al, 2017). Robertson et al (2012) demonstrated that women aged under 50 years with a TNBC had a greater than 10% likelihood of being a pathogenic BRCA variant carrier, irrespective of their family history. Their study found that testing only women aged under 40 years would miss 24% of pathogenic variant carriers (Robertson et al, 2012).
Since the introduction of BRCA gene testing, defined selection criteria have been continually evolving. Defined selection criteria to identify pathogenic BRCA variant carriers at any time point are based on the best available evidence at that time and, although these criteria have been extended in line with advances in knowledge and evidence, it is now clear that many carriers are not identified using such a selection criteria method. A recent study by Beitsch et al (2019) reported that nearly half of all patients with breast cancer with an actionable gene variant were missed by current testing guidelines. As a consequence of these findings, the American Society of Breast Surgeons' Consensus Guideline on Genetic Testing for Hereditary Breast Cancer now recommends that all patients with a personal history of breast cancer should be offered genetic testing (Manahan et al, 2019). Similarly, a recent UK study by Sun et al (2019) supported these findings and suggested that unselected, high-risk multigene testing for all patients with breast cancer remains cost-effective compared to testing based on family history or defined clinical criteria (Sun et al, 2019). This non-selective approach to gene testing is already being undertaken in many breast units outside the UK, including in the US, Iceland and Norway (Grindedal et al, 2017).
It is increasingly recognised that it is important to make genetic testing more readily available to newly diagnosed breast cancer patients as this may provide an opportunity for more effective and efficient treatment decisions, which could improve long-term health outcomes. Additionally, it may improve cancer risk information for unaffected family members and opportunities for predictive gene testing and risk-reducing interventions, which is a cost-effective cancer prevention strategy (Kwon et al, 2010).
Within the NHS, more accessible genetic testing has often been hindered by long waiting lists and limited access to genetics services, the high cost of genetic testing and complex referral pathways. The current standard cancer genetics pathway in the UK requires a patient to be referred to see a genetic counsellor or geneticist within a clinical genetics service that is separate from to the breast/ovarian oncology service in which their cancer is being treated (NICE, 2019). Genetic counselling has an important role in the interpretation of family medical histories, education about inheritance, management and ensuring patients have access to sufficient high-quality, evidence-based information to allow informed decision-making about genetic testing. The counselling sessions serve to open discussions around consent, testing, the implications of results and passing information to relatives. Genetic counselling also provides result-specific information with referral to other services where required.
Patients not affected by cancer who are undergoing predictive genetic testing, usually because of a family history, only have to attend appointments with their local clinical genetics service. Patients who have a breast cancer diagnosis and are undergoing diagnostic genetic testing will have appointments with breast oncology clinicians and clinical genetics services. When patients have to attend separate clinical genetics services, it can lead to additional appointments, longer waiting times and a more disjointed experience for them. By bringing genetic testing within the oncology clinics, the patient pathway is simplified and expediated (George et al, 2016). Genetic testing offered through the cancer oncology services rather than through separate clinical genetics services is referred to as mainstreaming cancer genetics (MCG).
Mainstreaming cancer genetics
In 2013, the Royal Marsden Hospital began a pilot MCG scheme in response to the problems associated with traditional gene testing, including the gene testing itself being slow and expensive, and the complex process for accessing gene testing. Before 2013, patients with cancer could access gene testing only if they were referred directly to a clinical genetics service. The referral criteria were complex and waiting lists were long.
The MCG pilot scheme at the Royal Marsden Hospital brought the genetic testing to the patients in the breast and ovarian oncology clinics rather than patients having to attend separate appointments with a clinical genetics service, often at a different hospital (Percival et al, 2016).
Following on from the MCG programme at the Royal Marsden Hospital, many UK hospitals implemented MCG services with different operating formats, including consultant-led, genetic counsellor-led and other multidisciplinary formats (George et al, 2016; Percival et al, 2016; Rahman, 2019). The benefits of MCG programmes have been demonstrated in the literature, with referral rates increasing, referral waiting times decreasing and genetic counselling appointment lengths reducing (Colombo et al, 2018). MCG programmes have also been shown to be more cost effective than traditional genetic testing pathways (Slade et al, 2016). In addition to these benefits, feedback questionnaires from 259 patients and 23 cancer clinical team members showed the process had been accepted, with 100% of patients pleased they had genetic testing and 100% of cancer team members confident to approve patients for genetic testing (Kemp et al, 2019).
Developing a nurse-led MCG programme
The Nottingham Breast Institute (NBI) and Nottingham Genetics Service are both part of Nottingham University Hospitals (NUH) NHS Trust. A nurse-led MCG service was developed and implemented within the NBI, which allowed patients diagnosed with breast cancer to access genetic testing during their breast clinic appointments rather than having to attend separate clinical genetics appointments.
In 2014, the NBI was diagnosing more than 800 breast cancers per year and the increasing number of women with TNBC eligible for BRCA gene testing was resulting in unmanageable workloads for the local clinical genetics service, which resulted in long waiting times for referral and test results.
The aim of the service change was to develop and implement a nurse-led, in-house MCG programme, which would reduce waiting times for an initial counselling appointment (which would be nurse-led), reduce the waiting time for results and to ensure all appropriate patients were offered genetic testing.
Methods
Overview
The service was initially designed to reflect the Royal Marsden Hospital MCG programme guidelines. The data collected were from the MCG database and included all patients who were having a diagnostic genetic test because they had a positive breast cancer diagnosis. This database does not include patients not diagnosed with breast cancer undergoing predictive genetic testing.
Two NBI breast clinical specialist nurses (with specialist training in breast family history) completed learning packages with local clinical genetics specialists who trained the nurses to obtain consent, counsel and give results for BRCA gene testing to patients.
The nurses were on NHS band 7 and had 30 and 40 years' NHS breast nursing experience. The two nurses worked with the genetics team to implement the MCG breast service within the NBI.
The clinical genetics service extended their training packages, competencies and protocols to implement MCG services trust wide at NUH; in addition to Nottinghamshire, NUH provides clinical genetics services and training services to surrounding areas including Mansfield, Grantham, Boston, Lincoln, Spalding, Skegness and Derby.
Training package
The breast MCG training package was developed by the NUH clinical genetics service and based on the Royal Marsden Hospital MCG training criteria, with input from the clinical nurse specialists at the NBI. The breast clinical nurse specialists who led the initial implementation of the breast MCG service in NBI have continued to train and mentor other breast care nurses through the breast MCG training package in conjunction with the clinical genetics service.
Self-directed learning
Trainees are required to self-complete basic background genetics learning, in the format of online YouTube videos produced by the Royal Marsden Hospital (MCG ELM1 and MCG ELM2), and read the Royal Marsden's beginner's guide to BRCA1 and BRCA2 (Royal Marsden NHS Foundation Trust, 2015).
NBI learning
Trainees are mentored by the MCG lead breast clinical nurse specialists, and complete patient observations, formal consent training and patient-facing genetics learning in practice. This consists of learning how to draw and interpret a three-generation family history tree, formal genetic consent training, practical counselling experience and acquiring knowledge needed to be able to answer patient questions before and after testing. Trainees are also taught to interpret, understand and deliver genetic test results to patients.
Competency is assessed through the MCG lead breast clinical nurse specialists observing trainees, documenting cases and professional dialogue, typically over a 6-month period. Consent training has a formally assessed competency.
Clinical genetics education
In parallel with the training within the NBI, trainees complete learning sessions with the clinical genetics service.
The clinical genetics education consists of using the Royal Marsden Hospital YouTube training videos MCG ELM3a and MCG ELM4 and completing a frequently asked questions pack. In addition, all trainees complete a formal face-to-face, half-day training session with the clinical genetics service to learn more detailed genetics science, how to refer patients with other cancer syndromes to the clinical genetics service and specialist genetics help for the practitioners in interpreting results that are unusual or difficult to interpret. This training session also teaches consent, counselling and knowledge about results in more depth. It consists of formal teaching and role play scenarios. Clinical genetics competency is assessed using a short answer test.
Ongoing learning
Nurses and other clinicians (mainly doctors) who attend the clinical genetics MCG training programme are expected to take responsibility to remain current with changes to genetic literature, testing protocols and personal CPD. The clinical genetics service maintains a register of trained clinicians and updates staff with genetic guidelines and protocol changes.
Regular liaison meetings are held between the clinical genetics service and the breast clinical nurse specialists to allow for ongoing training, discussion of changes to testing criteria and to share patient case studies.
MCG criteria
After the two breast clinical nurse specialists had been trained, a weekly clinic was set up as agreed with the multidisciplinary team (MDT) and the clinical genetics service. In 2015-2016, breast cancer patients who met the original mainstreaming criteria were offered an appointment with the breast clinical nurse specialists for counselling and consent.
The original mainstreaming criteria were:
All results were given to the patient by the trained breast clinical nurse specialist who, in addition to being family history specialists, are experienced breast care nurses. Genetic test results were also confirmed by letter following the nurse-led appointment and the breast MDT meeting. There was no longer a waiting period for clinical genetics to send the results to the clinical breast team as the clinical nurse specialists were able to provide the oncologists and surgeons at the MDT meeting with the patient's genetic test results as soon as they were available, ensuring efficient chemotherapy and surgical decision-making for each patient.
The clinical nurse specialists in the breast MCG service were able to directly refer patients to other services, including:
These referrals typically took 2 weeks.
The specialist nurses managed all the needs of newly diagnosed breast cancer patients with established pathogenic gene variants and were central in relaying information on family history and genetic testing results to members of the breast MDT.
The MCG pilot scheme at the Royal Marsden anticipated that, under the original mainstreaming criteria, 10% of patients tested would have a pathogenic genetic variant. In 2016, the NBI was operating the MCG service using the original mainstreaming criteria. Between 2017 and 2018, the MCG criteria at the NBI were extended to ensure a wider range of patients were eligible for genetic testing. The extended mainstreaming criteria were defined as follows:
Analysis
Descriptive statistics, tests for normality and independent t-test statistical analyses were performed using Stata 16 software.
Pre-MCG waiting time data for 2014–2015 are based on average service data. Figure 1 depicts the referral pathways for genetic testing before the nurse-led MCG breast service was introduced. Figure 2 shows the referral pathway for genetic testing via the nurse-led MCG breast service.
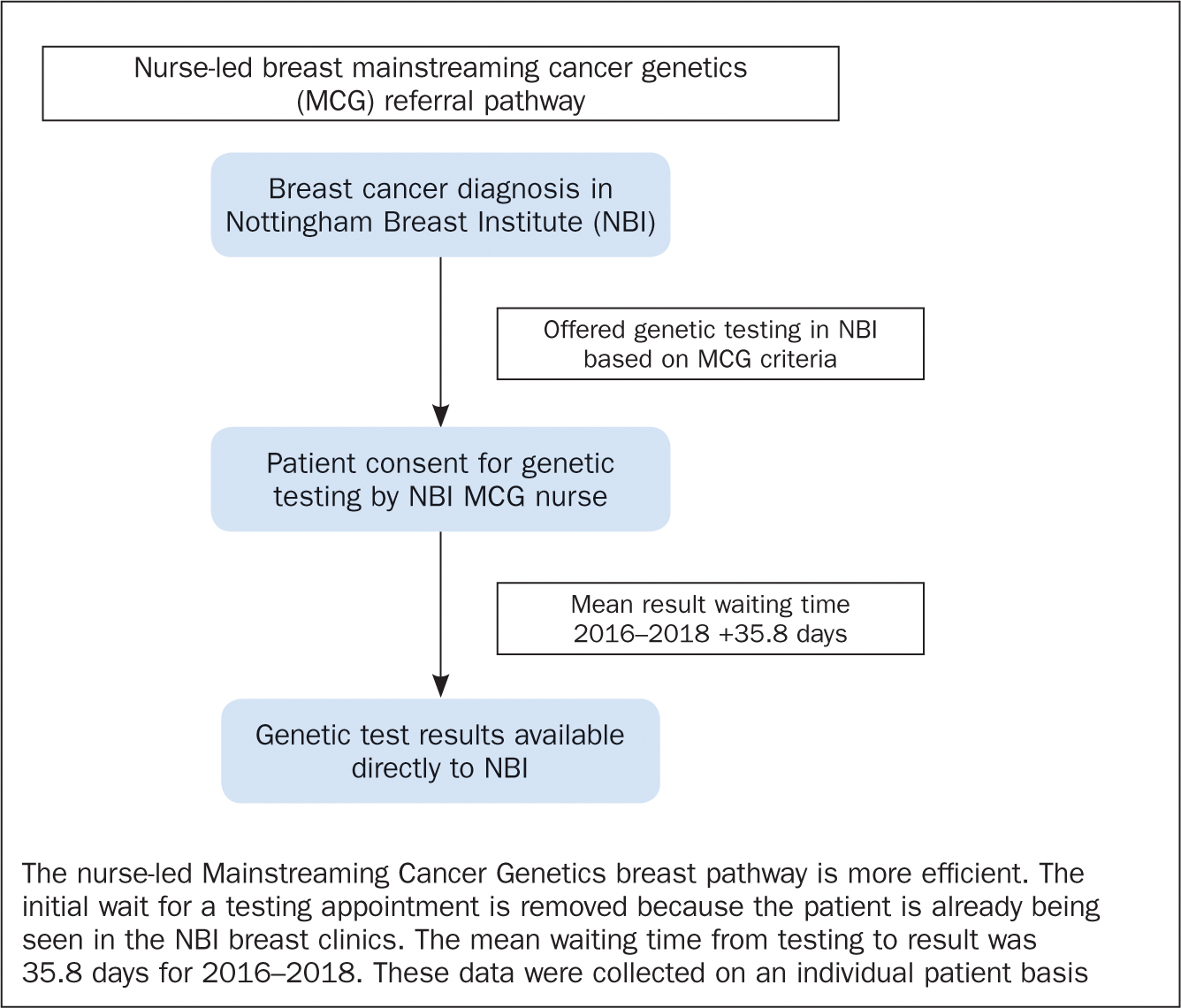
Original MCG criteria data (2016) and extended MCG criteria data (2017–2018) are based on individual patient data collected by the breast clinical nurse specialists in the MCG clinics.
Results
The data years 2016, 2017 and 2018 are full data years.
The total number of patients tested was 290 (2016, n=79; 2017, n=107; 2018, n=104).
In 2016, testing was based on the original MCG criteria, which were extended from 2017 (see above).
Table 1 shows that the mean age of testing in all years was under 50 years, with a minimum age of 23 years and a maximum age of 80 years.
| Year; number of patients tested | Mean age (years) | Minimum age (years) | Maximum age (years) |
|---|---|---|---|
| 2016; n=79 | 47.44 | 23 | 70 |
| 2017; n=107 | 49.81 | 29 | 70 |
| 2018; n=104 | 48.90 | 24 | 80 |
Table 2 shows that, over 2016-2018, the pathogenic variant testing rates were above 10% and there was an even distribution between BRCA1 and BRCA2 pathogenic variant results. In 2016, under the original MCG testing criteria, there was a 22.78% rate of pathogenic BRCA1 and BRCA2 variants. In 2017 and 2018, under the extended MCG testing criteria, the detection rates were 10.28% and 10.58%, respectively.
| Year; number of patients tested | No variant: n(%) | No variant mean age (years) | BRCA1 pathogenic variant: n(%) | BRCA1 mean age (years) | BRCA2 pathogenic variant: n(%) | BRCA2 mean age (years) | Unknown variant; n(%) | Unknown variant mean age (years) | Total BRCA1 and BRCA2 pathogenic variant: n(%) |
|---|---|---|---|---|---|---|---|---|---|
| 2016, n=79 | 58 (73.42%) | 46.69 | 9 (11.39%) | 48.00 | 9 (11.39%) | 50.89 | 3 (3.80%) | 50.00 | 18 (22.78%) |
| 2017, n=107 | 90 (84.11%) | 50.31 | 6 (5.61%) | 41.33 | 5 (4.67%) | 47.80 | 6 (5.61%) | 52.50 | 11 (10.28%) |
| 2018, n=104 | 90 (86.54%) | 49.08 | 5 (4.81%) | 40.20 | 6 (5.77%) | 47.17 | 3 (2.88%) | 61.67 | 11 (10.58%) |
Table 3 shows the differences between expected waiting times and actual waiting times for results for all 3 years. The reduction in waiting times following the introduction of the nurse-led MCG service was significant in all 3 years (t-test P<0.005 for all 3 years).
| Year; number of patients tested | Expected waiting time (days): mean (range) | Actual waiting time (days): mean (range) | Mean difference between expected and actual waiting times (days) |
|---|---|---|---|
| 2016; n=79 | 49.66 (4–98) | 37.56 (6–96) | −12.10 |
| 2017; n=107 | 46.93 (5–84) | 36.84 (6–62) | –13.09 |
| 2018; n=104 | 51.87 (30–77) | 39.61 (22–57) | –12.26 |
The average actual waiting time in all 3 years was 12.48 days less than anticipated. The average waiting time from the date of testing to the date of results in the three years was 35.8 days. Based on average service data, the waiting times in the 2 years before the nurse-led MCG service was introduced were 12–14 weeks (84–98 days) for the initial referral appointment to the clinical genetics service and 4–6 months to get results from the time of testing.
It should be noted that the clinical genetics service could fast-track results for patients where necessary, and the results here are based on average service data waiting times as individual patient waiting-time data were not being collected before the nurse-led MCG breast service was introduced.
Discussion
The move to a nurse-led MCG service has proved to be successful at the NBI. The aims of the nurse-led MCG service were to reduce waiting times for genetic testing appointments and results and to ensure all appropriate patients with breast cancer were offered genetic testing. The results demonstrate that waiting times for both the initial testing appointment and the time from testing to results were reduced significantly under the nurse-led MCG service, and extending the criteria of patients eligible for genetic testing in 2017 was justified as the extending the criteria still identified pathogenic genetic variants in more than 10% of the cohort tested.
Waiting time for initial testing was shortened significantly with an average waiting time from testing to results being less than 36 days. Waiting times in the 2 years (2014–2015) before the MCG nurse-led service was introduced were reported as being an average 12–14 weeks for an initial appointment with the clinical genetics service, and an average waiting time of 4-6 months for genetic test results after testing had taken place.
Because waiting times are shortened, treatment choices can be more patient-focused, which may improve patient outcomes. Obtaining a genetic test result before chemotherapy is started means that the chemotherapy can be tailored more specifically in pathogenic genetic variant carriers and surgical options can be discussed and considered before radiotherapy.
There is an argument that discussing genetic testing soon after a breast cancer diagnosis may be too much information for some patients (Ardern-Jones et al, 2005) and, as a result, patients may not fully understand the relationship between genetic testing and treatment decisions (Schwartz et al, 2018) and surgical decisions (Jacobs et al, 2019). In addition, qualitative evidence suggests patients with breast cancer are not always aware of how genetic testing can benefit them and, instead, view it as information provision for non-symptomatic relatives (Wright et al, 2018). The nurse-led MCG service was implemented to facilitate open discussion and understanding around genetic testing as the breast clinical nurse specialists are trained in genetics as well as being breast care nurses, and are able to guide the patient through decisions relating to all aspects of their breast care and clinical decision-making. Patients will be in contact with the breast clinical nurse specialists throughout their breast cancer treatment and can revisit genetic discussions at any point.
The clinical nurse specialists have become the central point of contact and information between the patients and the MDT teams. This has ensured that the surgeons, oncologists and wider breast MDT are aware of the genetic testing pathway of each individual patient. This has ensured better communication within the team and better continuity of care for patients throughout their breast cancer treatment.
Implications for nursing
The benefits of MCG services are well-evidenced in the literature regarding many NHS hospital trusts in the UK and numerous health services globally, but the evidence for nurse-led MCG services is minimal.
Using a nurse-led MCG service provides patients with continuity of care with a specialist breast care nurse, who will follow patients through their cancer treatment pathway. Having a specialist nurse-led clinical contact has long been successful in improving patient outcomes and patient satisfaction in other clinical areas such as cardiovascular medicine (Manoj et al, 2019; O'Toole et al, 2019), respiratory medicine (Sharples et al, 2002) and pain management services (Wells-Federman et al, 2002). A review that compared the effectiveness of nurse-led follow-up cancer services with conventional follow-up demonstrated a high level of patient satisfaction and improved efficiency and efficacy of clinical service delivery (Lewis and Hendry, 2009). Formal study data on patient satisfaction was not collected routinely as part of the MCG nurse-led service implementation in Nottingham, but informal data revealed that patients were happy with the service they received (through the NHS friends and family questionnaire), and patients expressed views showing they were pleased to be able to have genetic testing completed at their breast clinic appointments in the NBI. Research plans include a formal service evaluation, which will include collection of both quantitative and qualitative patient experience data.
In Nottingham, increasing demand on breast care services has been exerting an increasing burden on outpatient, breast care and oncology follow-up services. The day-to-day delivery of the nurse-led MCG service has been implemented by breast clinical nurse specialists in conjunction with the local clinical genetics service, which allowed the nurses to gain experience of service development at trust level. Using a nurse-led MCG service has given nurses a greater therapeutic role and the opportunity to gain additional clinical experience. In addition, it has extended the role of breast care nurses within the MDT and allowed them autonomy, responsibility and accountability for developing and improving the service, and has included mentoring nurse trainees through the training package and evaluating the evidence to extend MCG testing criteria within the NBI. The nurses have accountability over the data being collected in the NBI BRCA database and ensuring that it is accurate, thus supporting the NBI to perform high-quality research. These opportunities serve to advance the nursing clinical practice experience, which is increasingly important for Nursing and Midwifery Council revalidation.
The nurse-led MCG service has close links with the family history risk-reducing surgery clinics at the NBI. Both female and male biological relatives of BRCA-positive breast cancer patients are given the opportunity to have genetic testing through the clinical genetics service, and any female relatives found to be carrying the pathogenic BRCA variant are given the opportunity to be referred to the risk-reducing breast surgery clinics at NBI. These clinics are supported by the same clinical breast nurse specialists as the MCG service. The nurse specialists also organise patient support groups, which have both an educational component and a discussion format based on all aspects of BRCA genetic testing and decision marking.
This full cycle of nurse-led care allows for continuity of care not just for individual patients but also within families undergoing breast cancer treatment, genetic testing and risk-reducing breast surgery.
Future of MCG in Nottingham
The nurse-led MCG service in Nottingham has been deemed to be successful and, as a result, testing for the pathogenic PALB2 genetic variant has been added to the genetic testing panel.
It is likely that more extensive and non-selective (all newly diagnosed breast cancer patients) gene panel testing will occur in the NHS in the future, based on evidence from recent publications (Grindedal et al, 2017; Manahan et al, 2019; Sun et al, 2019). This will have major implications for service delivery and restructuring of breast services in the future, and breast clinical nurse specialists trained in genetic counselling will play a central role in the way these changes are translated into clinical practice. Within the NBI, there is now an expectation that all newly recruited breast nurses will be trained to be involved with the MCG service, which future-proofs the service in anticipation of the predicted widening of testing criteria and consequent increase in demand.
The NBI and clinical genetics service will continue to ensure appropriate development of the service in line with genetic guideline changes and advances, for example supporting the implementing changing guidelines in the National Genomic Test Directory (NHS England, 2019), and following the work of Cancer Research UK's CanGene-CanVar national research project, which aims to improve patient care in relation to clinical genetics (http://cangene-canvaruk.org).
Conclusion
The MCG service in Nottingham has been successfully implemented in a nurse-led format with the training of specialist breast care nurses. This has reduced waiting times for consent and results appointments. Management of the MCG service by nurses has seen MCG testing criteria expand, which has ensured that more breast cancer patients have been tested for pathogenic genetic variants. It is likely that these specialist nurses will play a key role in the delivery of breast services of the future as genetic testing will evolve and increase dramatically.

