Pain is an integral part of life that is experienced in one way or another by virtually everyone at some time in their life and it is often the reason why medical help and advice is sought. It can be caused by a simple bump, a complex disease process or a surgical intervention. It can often be self-managed but there are many instances where medical and nursing interventions are required. These interventions may be as straightforward as the administration of analgesia to an inpatient following surgery, or they may involve careful multi-modal assessment and interventions by a specialist pain team.
Pain can be defined in a several different ways. In this article two definitions will be used. The first is the international standard from the International Association for the Study of Pain (IASP) and the second is a definition that is much more relevant to clinical practice.
The IASP (2023) defines pain as ‘an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage’. In contrast, McCaffery (1972) used a clinical approach and said pain was ‘whatever the person experiencing it says it is and exists whenever he says it does’.
The IASP (2023) and Raja et al (2020) went on to qualify their definition with a number of explanatory notes that clearly support the concepts identified by the McCaffery definition: that pain is a personal experience and is subjective. The inclusion of these explanatory notes gives a flavour of the complexity of pain, making a simple all-encompassing definition impossible at present. The subjectivity of a pain experience is also highlighted as well as the biopsychosocial context. In simple terms, healthcare staff cannot know as much about a patient's pain as the patient does. In addition, healthcare staff should believe the patient when they say they are in pain and respect their account of their pain experience. If the complaint of pain seems inappropriate or excessive, then healthcare staff should seek to identify the reasons behind this and, where possible, use their skills to relieve it using the variety of means available.
Marieb and Hoehn (2019) explained that there are emotional, psychological, social, cultural, and experiential influences on an individual's understanding of, and response to, pain. This suggests that an individual's understanding of and response to pain is learned through experiences of it and conditioned by those experiences. Past experiences might lead to under-reporting of pain: a former soldier feeling ashamed of admitting to a male nurse that he has excruciating pain may not report his pain in a timely or accurate manner; someone perceiving a nurse to be busy may also play down their pain. Nurses therefore need to understand pain, its assessment and management.
The aim of this article is to introduce health professionals to the different types of pain and the associated pathophysiology, particularly the nociceptive and neuropathic aspects but with a brief mention of newer ideas such as mixed pain, nociplastic pain and the neuromatrix. It will then look at a variety of assessment tools across the lifespan and ability span before concluding with pharmacological and non-pharmacological methods of management.
Categories of pain
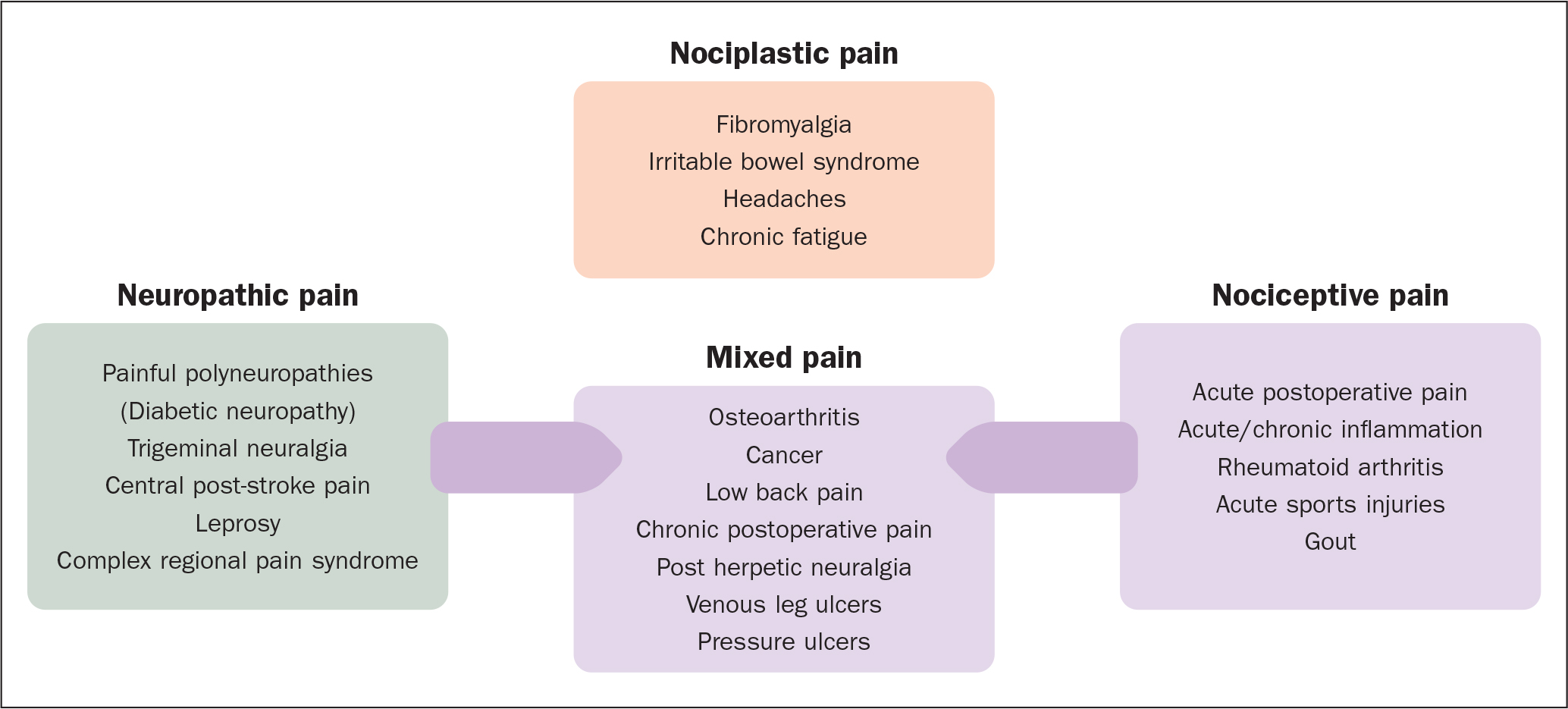
The IASP divides pain into three categories (Box 1). Nociceptive pain is associated with damage to non-neural tissue and involves the stimulation of nociceptors (pain receptors attached to sensory neurones). It is often referred to as a protective device and serves as warning of actual or impending tissue damage. This type of pain is one of the four classic manifestations of the inflammatory response. It is important to remember that there is no directly proportional relationship between the degree of injury and the expression of pain. Whether using a more traditional approach or the concept of neuromatrix adjustment, our perception of pain is modulated by our experiences and therefore each individual will respond differently to an injury.
Box 1.Categories of pain
- Nociceptive pain: pain that arises from actual or threatened damage to non-neural tissue and is due to the activation of nociceptors
- Neuropathic pain: pain caused by a lesion or disease of the somatosensory nervous system
- Nociplastic pain: pain that arises from altered nociception despite no clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system causing the pain
Source: International Association for the Study of Pain, 2023
Nociceptive pain is often divided into somatic and visceral (McCance and Huether, 2019). Somatic pain is associated with the integumentary and musculoskeletal system and tends to produce sharp, localised pain. Visceral pain is associated with the contents of the thoracic, abdominal and pelvic cavities. This tends to be associated with dull, diffuse pain although the endothelium of the gut can have acute localised pain.
Neuropathic pain is associated with damage to neural tissue in either the central or peripheral nervous system (IASP, 2023). Macintyre and Schug (2021) suggested that although neuropathic pain is usually associated with chronic pain it can also be acute, later transforming into chronic pain. Neuropathic pain should always be suspected where there is nerve damage. Peripherally it may be manifested when nerves are transected at amputation or where there is significant tissue loss, as in category 3 and 4 pressure ulcers. Centrally a stroke will lead to damage to central neurones, which may also result in neuropathic pain (central post-stroke pain) (Stroke Association, 2017).
Nociplastic pain is a relatively new addition to nociceptive and neuropathic pain. Fitzcharles et al (2021) described this pain as multifocal, and highlighted that it may also be more widespread or intense than would be expected by the clinical picture. There are often other central nervous system symptoms such as fatigue, sleep, and mood problems. Nociplastic pain can exist alone, but it will also exist in conditions such as fibromyalgia and tension-type headaches.
Freynhagen et al (2019) suggested that a new definition called ‘mixed pain’ should be developed and added to the three current IASP categories. Fitzcharles et al (2021) stated that more than one pathophysiological pain process may well be manifested at the same time. In their terms there may be nociceptive and neuropathic features or possibly neuropathic and nociplastic elements. There may also be manifestations of acute and chronic pain as in rheumatoid arthritis or when an individual undergoing surgery also has an unrelated chronic painful condition.
Pathophysiology of pain
The types of pain identified in Box 1 indicate the main characteristics of the pathophysiology involved in the manifestation of that type of pain. Nociceptive pain arises as a consequence of the inflammatory process in non-neurological tissue and involves the process of nociception. Neuropathic pain arises as a result of damage to the neurones of the central or peripheral nervous system. Nociplastic pain arises as result of changes in nociception without any demonstrable pathology. It has been noted that the pain experience can be modulated by a number of biopsychosocial factors and that some conditions and disease processes can manifest more than one type of pathophysiological process.
In addition, pain has been described as acute or chronic (Jenkins, 2020a; 2020b). Acute pain is considered to become chronic once it has persisted for more than 12 weeks. It may also be referred to as non-remitting or remitting — that is to say, whether it is continuous or intermittent. Although these and other historical categories of pain can be subsumed into the current theoretical thinking, they are still found in common speech and are understood by most people, therefore should be part of the health professional's vocabulary.
Nociception
Nociception is the process starting with a noxious stimulus and ending with the perception of pain, which may then be further modulated. McCaffery and Pasero (1999) described the process, identifying four stages: transduction, transmission, perception and modulation. In simple terms transduction is the conversion of one type of energy into another. All the stimuli need to be converted into the electrical energy of the neuronal action potential.
Transmission follows the three-neurone sensory pathway. The peripheral neurone runs from the receptor through the dorsal root ganglion into the spinal cord where it synapses with the spinal neurone in the substantia gelatinosa of the dorsal horn. The spinal neurone then crosses over and ascends via the spinothalamic tract to the thalamus, where it synapses with the third neurone that travels on to the somatosensory cortex (Marieb and Hoehn, 2019).
Perception is characterised by conscious awareness of pain once it has reached the somatosensory cortex. Briggs (2010) and Marieb and Hoehn (2019) identified that perception is influenced by experience, memory and emotion through the limbic system and the reticular activating system, which includes the thalamus itself and where virtually all sensory inputs synapse and make multiple connections with a variety of centres in the brain including the somatosensory and motor cortices, areas of the limbic system and the frontal, cognitive cortex.
Modulation is the ability to influence and change the pain signals. This may be by inhibiting transmission or by altering the emotional response to pain for example. The effects take place mainly in the central nervous system, although a number of peripheral inputs such as transcutaneous electrical nerve stimulation (TENS) or ‘rubbing it better’ can be involved. Using the neuromatrix model, the central nervous system acts to correct a deficit based on previous learning and experience to influence the response to the insult (Institute for Chronic Pain, 2017)
Nociceptive pain
Nociceptive or inflammatory pain is induced by a range of chemicals that are formed or released following non-neural tissue injury. Damage to cell walls leads to the formation of arachidonic acid under the influence of the enzyme phospholipase A2(Figure 1). Arachidonic acid is then converted into two groups of chemicals that can stimulate or influence the pain receptors. One group of chemicals, the prostaglandins, are produced under the influence of cyclooxygenase (McCance and Huether, 2019). These have a hyperalgesic and vasodilatory effect. The production of prostaglandins is influenced by the presence of bradykinin, which is produced when cells are damaged. Once produced the prostaglandins lower the nociceptor threshold thereby enhancing the action of both bradykinin and 5-hydroxytriptamine (serotonin) on the nociceptors and increasing pain perception, causing primary hyperalgesia (Ritter et al, 2018). The second group of chemicals produced under the influence of5-lipoxygenase are the leukotrienes. Only one of these is inflammatory and of relevance to the induction of pain (Ritter et al, 2018).
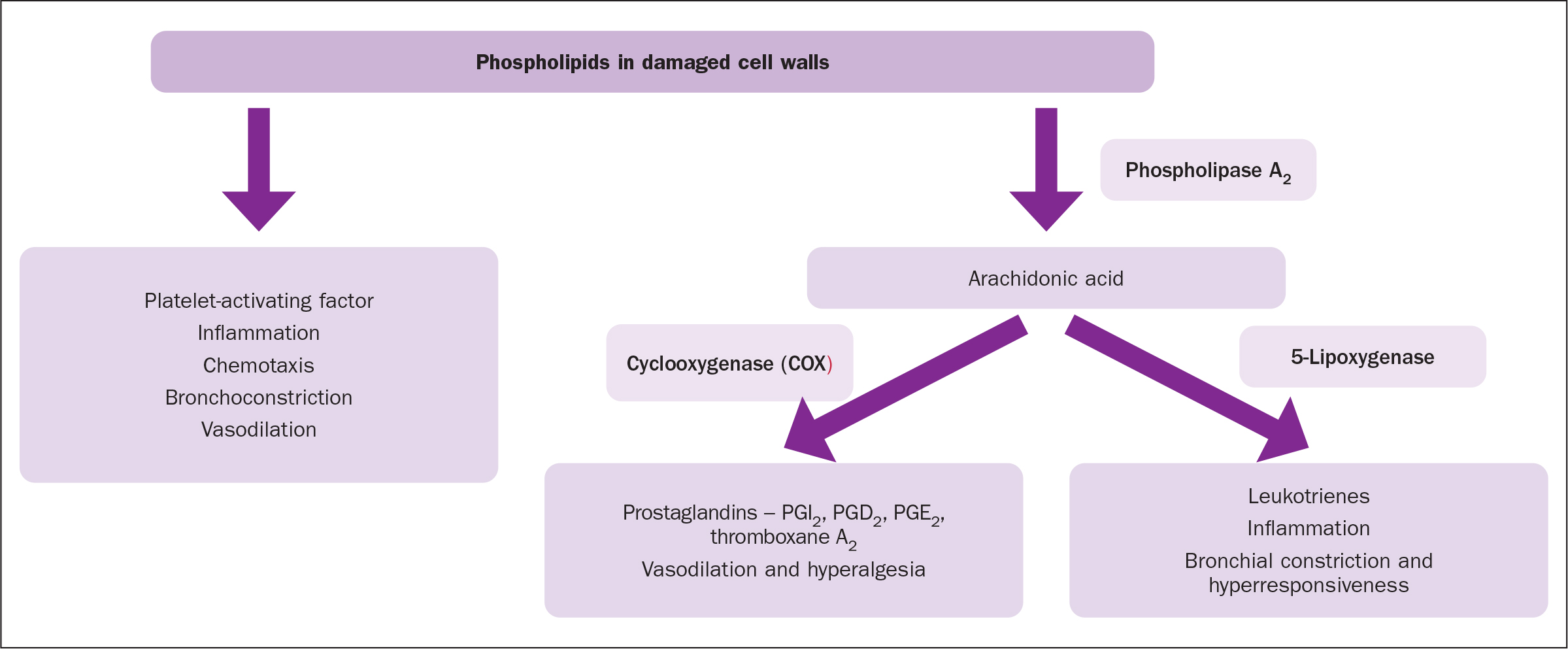
Nociception involves two types of free nerve endings: high-threshold mechanoreceptors and polymodal nociceptors. The polymodal nociceptors in particular are able to react to a range of noxious stimuli, including mechanical, thermal and chemical. The high-threshold mechanoreceptors respond to mechanical and thermal stimuli (Ritter et al, 2018). The two nociceptors are associated with specific types of neurone. The high-threshold mechanoreceptors are associated with A delta (Aδ) neurones, these have moderate diameter axons and thin myelination conducting at 6-30 metres per second (m/s). The polymodal receptors are associated with C neurones, which have thin axons, no myelin and conduct at 1.0-2.5 m/s. Compare this with gentle touch/pressure fibres found in the skin that have thicker axons and myelin coat, A beta (Aß) neurones, which can conduct action potentials at 30-180 m/s (Briggs, 2010).
Neuropathic pain
Identifying neuropathic pain is important as its management is significantly different from that of nociceptive pain.There are several changes that take place because of damage to nervous tissue. Briggs (2010) and Macintyre and Schug (2021) considered some changes that take place resulting in the manifestation of neuropathic pain.
The signs and symptoms of neuropathic pain are variable and unpredictable but there are common features: ectopic and spontaneous action potentials producing random or repeated pain signals, changes in skin temperature and colour, along with changes in skin sensation. Paraesthesia and numbness are also common features. Secondary hyperalgesia, where the uninjured area around an injury has an exaggerated response to a painful stimulus, is a possible consequence. Allodynia may be manifested — in this situation there is an exaggerated pain response to a non-painful stimulus (Finnerup et al, 2021).
These manifestations arise due to changes caused by the damage to the neurones and nerves. Where nerves are transected, there is a change in the membranes of the neurones around the area of damage. Stable sodium channels are replaced by less stable channels, leading to stimulus-independent membrane depolarisation. This can also be manifested in uninjured nerves as electric shock-type pains or shooting pains. At the ends of damaged nerves there may be movement of action potentials between neurones as opposed to along them, potentially leading to misinterpretation of signals. In addition, there may be changes in the dorsal horn and dorsal root ganglion with neurones making new connections by sprouting new branches (Xu and Yaksh, 2011)
Nerve damage also leads to an increase in the receptive field, which can lead to a larger area being sensitised. There is also a death of inhibitory interneurons and an increase in excitatory neurotransmitters in the central nervous system, both of which will increase the pain experience (Xu and Yaksh, 2011).
Figure 2 describes the common causes of pain.
Assessment of acute pain
Valid and reliable pain assessment tools are vital for good clinical care to ensure accurate and reproducible results to get the best outcomes for patients (Gordon, 2015). One of the problems, however, is that pain is a complex phenomenon with more than one dimension. Often, the assessment tools that are used in clinical settings are unidimensional, measuring only the intensity of a patient's pain. Macintyre and Schug (2021) highlighted the need for a multidimensional approach with an initial emphasis on taking a structured pain history. Problems arose in the USA when analgesic doses were adjusted only on patient-reported pain scores, particularly with opioid-induced ventilatory impairment (a more precise description of respiratory depression) in what has become known as the US opioid crisis (Compton et al, 2021). Obviously other side-effects such as nausea and vomiting, constipation and addiction were also increased with increasing dosage and prolonged activity.
Gordon (2015) suggested the use of the Clinically Aligned Pain Assessment Tool (CAPA) (Table 1). Macintyre and Schug (2021) took a slightly simpler approach using a three-point functional activity score (Table 2). The score must be assessed relative to the patient's baseline functional ability and should be based on activity relevant to the cause of the acute pain.
Table 1. Clinically Aligned Pain Assessment Tool
| Domain | Response |
|---|---|
| Comfort |
|
| Change in pain |
|
| Pain control |
|
| Functioning |
|
| Sleep |
|
Source: Gordon, 2015
Table 2. Functional Activity Score
| Score | Comments |
|---|---|
| A | No limitation of relevant activity |
| B | Mild limitation of relevant activity |
| C | Severe limitation of relevant activity |
Source: Macintyre and Schug, 2021
The key point both are promulgating is that simple unidimensional assessment is not sufficient. As identified above a functional assessment is considered a gold standard approach. Macintyre and Schug (2021) included consideration of a range of pain intensity scores, as well as specially adapted scoring tools for individuals with cognitive impairment and limited language skills. They also consider assessment tools for the assessment of neuropathic pain
There are several simple intensity scales in use. Probably the three best known are the Visual Analogue Scale (VAS), Numerical Rating Scale (NRS) and Verbal Rating Scale (VRS). There are a variety of versions of these available.
The VAS consists of a single 10 cm line with one end marked as ‘no pain’ (or similar), and the other ‘worst pain imaginable’ (or similar). This is a widely used tool as it quick and easy to use. It does, however, present problems particularly with older individuals and those with cognitive impairment that were identified some time ago (Hawker et al, 2011). The NRS and VRS are self-explanatory but again there are variations and problems (Hawker et al, 2011)
Most of the multidimensional tools are considerably more complex, with most having been designed for research rather than day-to-day clinical use. One exception is the short form of the McGill Pain Questionnaire (S-F MPQ) (Melzack, 1987) updated in 2009 to include neuropathic assessment as McGill short-form 2 (S-F MPQ-2) (Dworkin et al, 2009). Both assessment tools require training for the user and demonstrate some of the problems identified by Hawker et al (2011).
A range of pain assessment tools is available for individuals who have cognitive or developmental problems and who have not developed language or who have language difficulties. One of the best known of these is the Wong-Baker faces (http://www.WongBakerFACES.org). There are a number of iterations of the tool, from the Wong-Baker FACES Foundation and also from third-party producers. Following questions about possible misinterpretation, in 2001 the IASP produced a revised version of the Faces Pain Scale (FPS-R), which changed the smiley face at ‘no pain’ to a neutral face and removed the tears from the image for ‘worst pain’. This was done in attempt to separate nociception from other emotional states including fear and non-nociceptive stress (IASP, 2001).
For babies and infants there is a range of tools that use physiological and psychosocial cues to assess degrees of pain. These include the FLACC Scale (face, legs, activity, cry, consolability) (Merkel et al, 1997). Brand and Thorpe (2016) suggested the use of the QUESTT mnemonic (Box 2) to give a systematic approach to the assessment of pain in children.
Box 2.The QUESTT approach to pain assessment in childrenQuestion the childUse the age and developmentally appropriate scaleEvaluate behaviour and physiological changesSecure parental involvementTake the cause of pain into accountTake action and evaluate resultsSource: Brand and Thorpe, 2016
An area that raises many concerns is the assessment in individuals who are cognitively impaired, particularly people with dementia. IASP (2021) identified a number of assessment tools for people with dementia including: Pain Assessment in Advanced Dementia (PAINAD), Pain Assessment in Impaired Cognition (PAIC) and Pain Assessment Checklist for Seniors with Limited Ability to Communicate (PACSLAC). These scales, like those for the very young, rely heavily on physiological, emotional, and psychosocial indicators. The IASP in the same publication also make it clear that, wherever possible, self-reporting of pain should be valued and encouraged and that, particularly in moderate to severe dementia, clinicians should not take a lack of self-report as an absence of pain.
Treatment and management of acute pain
A number of writers have clearly indicated the need for careful holistic or biopsychosocial assessment of patients prior to beginning treatment (Gordon, 2015; Ritter et al, 2018; Macintyre and Schug, 2021). A feature of the recommended assessment is to identify the specific functional loss and to tailor the pharmacological interventions to maximise the specific functionality identified as well as reducing the intensity of the pain.
Table 3 gives a review of the main pharmacological interventions for the management of acute nociceptive and neuropathic pain. Humans produce their own pain inhibiting chemicals called endorphins. These are also referred to as endogenous opioids and are produced in response to painful stimuli. They inhibit the same receptors as the exogenous opioids that are administered as analgesics (Ritter et al, 2018).
Table 3. Pharmacological management of acute pain
| Pathophysiology | Medicine groups | Medications | Mode of action |
|---|---|---|---|
| Acute nociceptive pain | Cyclooxygenase inhibitors |
|
Act at the site of injury. By inhibiting the production of prostaglandins, the interaction between them and bradykinin is reduced and therefore there is less pain |
| Opioids |
|
Inhibit the transmission of impulses in the central nervous system via specific opioid receptors | |
| Combined cyclooxygenase inhibitors and opioids |
|
See above | |
| Acute neuropathic pain | Opioids* |
|
Inhibit the transmission of impulses in the central nervous system via specific opioid receptors |
| Gabapentinoids |
|
Inhibition of specific central calcium channels and serotonin pathways | |
| Tricyclic antidepressants |
|
Probable inhibition of nor-adrenaline and serotonin reuptake and N-methyl-D-aspartate (NMDA) inhibition | |
| Serotonin and noradrenaline re-uptake inhibitors (SNRIs) | ■ Duloxetine | Inhibits the re-uptake of both serotonin and noradrenaline | |
| Anaesthetic | ■ Ketamine† | Probable N-methyl-D-aspartate (NMDA) inhibition |
*Limited use, observe for increased sedation or dose without concomitant pain relief (Macintyre and Schug, 2021)
†Not listed as a first-line treatment for neuropathic pain (Joint Formulary Committee, 2022; National Institute for Health and Care Excellence, 2021; 2022)
Non-pharmacological interventions
Schug et al (2020) in their review of acute pain management indicated that there is some evidence to support the use of acupuncture and TENS, particularly in postoperative situations. Similarly, there is some evidence to suggest that physical therapies such heat and cold treatments can be effective. Where there is significant soft tissue damage the PRICE (protection, rest, ice, compression and elevation) approach to management and reduction of inflammation is an effective strategy (Norton, 2016).
Macintyre and Schug (2021) identified several psychological strategies that may be useful in managing acute pain. These include hypnosis, distraction including guided imagery, relaxation techniques and cognitive behavioural interventions.
The giving of information to alleviate pain is an essential skill that has been recognised for many years (Hayward, 1975). It remains a very significant tool in the alleviation of acute pain today (Gregory, 2019). There are resources available such as ‘ Understanding and Managing Long Term Pain: information for people in pain (British Pain Society, 2015) to help both professionals and patients to understand the experience of pain.
Case study
Julia, a 72-year-old lady, had been treated with high-compression bandaging for a venous leg ulcer for 6 months. Up until the last visit, she had been completely pain-free but now she was complaining of sharp pain, soreness and tenderness in and around her ulcer. When she first complained about the pain her community nurse carried out an assessment that identified that there was redness around the margin of the ulcer and a numerical pain score of 4 but with little effect on Julia's activity. The nurse suggested paracetamol 1 g no more than every 4 hours. This was reviewed after 2 days. On reassessment, Julia stated that if anything the pain was worse, scoring 5 on the numerical assessment tool. It was also beginning to affect her walking and ability to carry out her usual activities. The nurse escalated to Julia's GP, using the SBAR framework (situation, background, assessment, recommendation), recommending a prescription of codydramol, which she knew contained paracetamol and codeine and should therefore be more effective in controlling Julia's pain than paracetamol alone.
Unfortunately, after 3 days with the new prescription the pain was still present and becoming more persistent. The nurse again assessed the situation. This time Julia identified the pain as burning with prickling and itching in and around the wound. To double-check her conclusions the nurse used the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) chart for neuropathic pain (Bennett, 2001), which clearly indicated that there was a neuropathic element to Julia's pain.
From Julia's initial assessment when she was first diagnosed the community nurse identified that Julia was an avid reader of historical fiction. She used this knowledge to suggest that Julia tried reading to distract her from the pain. She also suggested that Julia kept her leg elevated when sitting and try to keep her toes warm, which would help keep her leg warmer and might relieve her pain. The nurse also contacted the GP, again using the SBAR format, this time recommending the prescription of pregabalin for Julia.
After 2 days Julia reported much less pain, which was easily managed by paracetamol as required. She was continuing with her reading and now had a fleece foot muff to keep her toes warm because these two approaches helped to ease her pain.
Conclusion
By understanding the physical manifestations of acute pain and having the ability to differentiate between the types of pain identified in this article the community nurse was able to identify that Julia probably had acute inflammatory nociceptive pain. She was aware that the pain intensity was such that paracetamol, and a mild opioid should manage the pain. In this way the pain could be managed at the point of injury by reducing the activity of prostaglandins and centrally by the dampening of the pain signals in the central nervous system. The nurse was also aware that combining pharmacological and non-pharmacological methodologies could improve the outcome. Again, when informed of the change in the quality of the pain, the community nurse was able to use her knowledge of pain pathophysiology to assess for the presence of neuropathic pain and make an appropriate escalation to the GP.
Key points
- Pain is a subjective experience and therefore cannot be objectively measured
- Nociceptive pain is part of the inflammatory process that affects non-neural tissue and neuropathic pain is the result of damage to neural tissue
- Patients may have more than one type of pain at the same time therefore health professionals should be aware of the key features of each type of pain
- It is important to choose a validated pain scale that matches the patient's capabilities and symptoms to ensure the best possible assessment
- Health professionals should work with patients to obtain concordance in the assessment and management of pain
CPD reflective questions
- What could be done to enhance patient pain assessment in your clinical setting?
- How could non-pharmacological pain management be developed in your clinical setting?
- Reflect on your current knowledge and practice and identify aspects you could develop to improve patient-centered care
- Using knowledge gained from this article and further study write a revalidation reflection on managing a specific patient's pain


