Disorders of the renal system, including kidneys and urinary tract, are estimated to account for 830 000 deaths each year worldwide and are the 12th biggest cause of death (Jager et al, 2019). Renal disorders cover a range of diseases with different aetiologies, trajectory, functional severity and treatment options, ranging from minor changes in renal function to more serious conditions including acute kidney injury or, if present, in the long term, chronic kidney disease. These patients also tend to present with other comorbidities such as hypertension, diabetes and cardiovascular disease. These are also predictors for the occurrence of acute kidney injury, further aggravating prognosis and mortality outcomes.
When not managed, renal diseases can progress to end-stage renal disease (ESRD) whereby kidney function must be substituted by renal replacement therapy (RRT): haemodialysis, peritoneal dialysis or, when patients fit the selection criteria, transplantation. However, patients are more likely to die from comorbidities, such as cardiovascular causes, than reach ESRD with dialysis intervention, illustrating the complexity of this cohort of patients and the need to manage comorbidities to allow better renal function (Jankowski et al, 2021). Considering the adverse impact of renal disease on public health, awareness of the severity and risks of these conditions is important for nursing practice. Nursing care for this comorbid cohort of patients is challenging as patients can deteriorate quickly. Understanding the different classifications, interventions and the ability to create tailored care plans, is essential to deliver patient-centered care, aiming to improve health-related outcomes.
The renal system: an overview
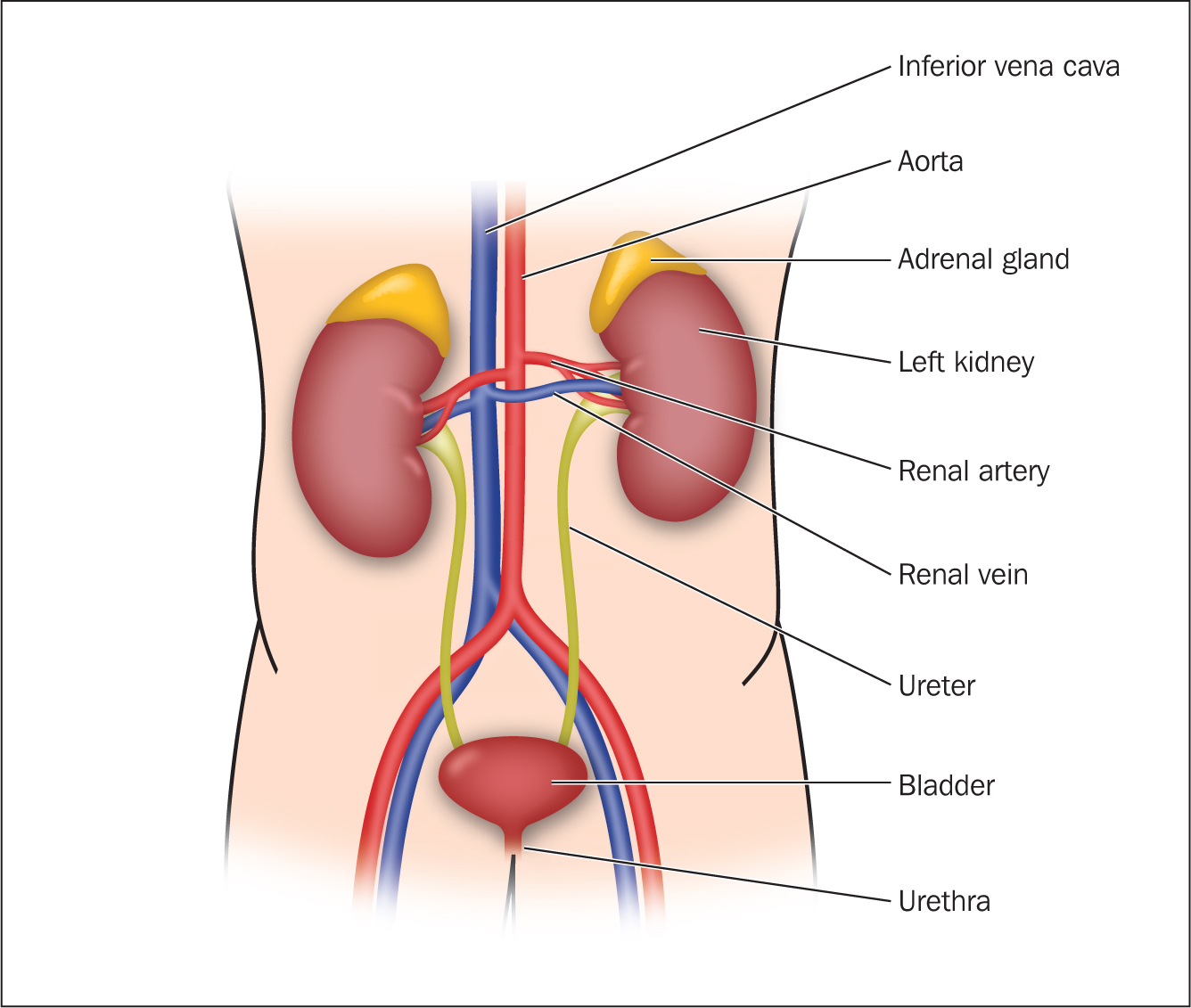
The renal system plays an important role in homeostasis and its function is to filter approximately 200 litres of fluid each day and allow excretion of toxins and metabolic waste while keeping essential substances in the blood, supporting electrolyte balance. It is composed of the kidneys, ureters, bladder, and urethra (Figure 1). The kidneys regulate the volume and composition of extracellular fluid, removing waste and extra fluid from the body and assisting control of blood pressure, among other functions (Box 1).
Box 1.Functions of the kidney
- Fluid homeostasis
- Excretion of urea and creatinine (nitrogenous waste)
- Electrolyte homeostasis (potassium and sodium)
- Secretion of renin (control of blood pressure)
- Production of red blood cells (erythropoiesis)
- Acid–base balance
- Synthesis of vitamin D
- Detoxification
- Gluconeogenesis (generation of glucose from certain non-carbohydrate carbon substrates)
The two kidneys are found at the back of the abdomen on the posterior wall (retroperitoneal), usually 5–6 cm wide and 3-4 cm thick. The outer border of the kidneys is convex, and the inner border is known as the hilum. The outer capsule of the kidney, called the renal capsule, protects the kidney from damage (Ashelford et al, 2019). It is here that renal arteries, renal veins, nerves and ureters enter and leave the kidneys (Nagalingam, 2021). The kidneys have a rich blood supply from the aorta via the renal artery with approximately 1200 ml of blood flowing through each kidney every minute. Nephrons are situated within the medulla; their main function is to produce urine, which is drained into the tiny ducts.
Urine concentration is controlled by the hypothalamus and the posterior pituitary gland and when an increase in blood osmolality occurs (increased concentration of blood particles), anti-diuretic hormones are released, causing water reabsorption in the kidneys and more concentrated urine. Characteristics of urine change according to a wide range of factors such as fluid or nutrient intake, age, body mass index, exposure to exercise, and environmental temperature.
Urine leaves the kidneys through the ureters, draining into the bladder. The sensory nerve fibres in the bladder wall signal the brain to trigger the process to expel the urine, which is known as micturition, with the bladder in healthy adults holding an average of 500–600 ml of urine before voiding (Waugh and Grant, 2018).
Measuring normal kidney function
Laboratory tests
Monitoring and establishing kidney function through biomarkers allows health professionals to define the parameter of structural, chemical, or physiological change that suggests the presence, severity or progress of a disease (Wasung et al, 2015). However, evidence suggests these biomarkers are not powerful in detecting the early stages of renal disease as kidney injury starts with biological and molecular changes, evolving into cellular damage at a later stage when these would be able to be measured in the blood (Wasung et al, 2015). Kidney function is usually measured through creatinine levels in the blood, or ‘serum creatinine’ (SCr), and blood urea nitrogen (BUN). Another commonly used test, glomerular filtration rate (GFR), is not a biomarker but an estimation of the clearance of filtrate in the glomerulus (Table 1).
Table 1. Renal function laboratory tests
| Serum creatinine level | Creatinine is the end product of protein metabolism and wear on muscles, directly affected by muscle mass. The more muscle, the higher the creatinine level in the blood. Normal creatinine levels in adults are 59–104 umol/litre for males and 45–84 umol/litre in females. |
| Blood urea nitrogen test (BUN) | Measures the nitrogen component of urea in the blood, it drops as eGFR drops and can be affected by other factors than renal disease such as malnutrition, sepsis, heart failure, hypovolaemia (Seki et al, 2019) |
| Estimated glomerular filtration rate (eGFR) | Calculation through a formula using serum creatinine, age, gender and body mass index. Generally, normal GFR is above 90 ml/minute/1.73m2 |
Urine tests
Urine output remains a powerful early indicator of kidney injury as to successfully excrete body wastes, an adult must produce around 1 ml/kg or 0.5 ml/kg/hour of urine in relation to their body weight (Waugh and Grant, 2018). Changes in urine characteristics and output can also inform objective history taking and management plans, as these can suggest renal disease. Urinalysis is performed in two parts. The first, usually done in a quick manner when abnormalities are suspected, consists of dipping a reagent strip into urine and noting color changes in each section of the strip (Figure 2).
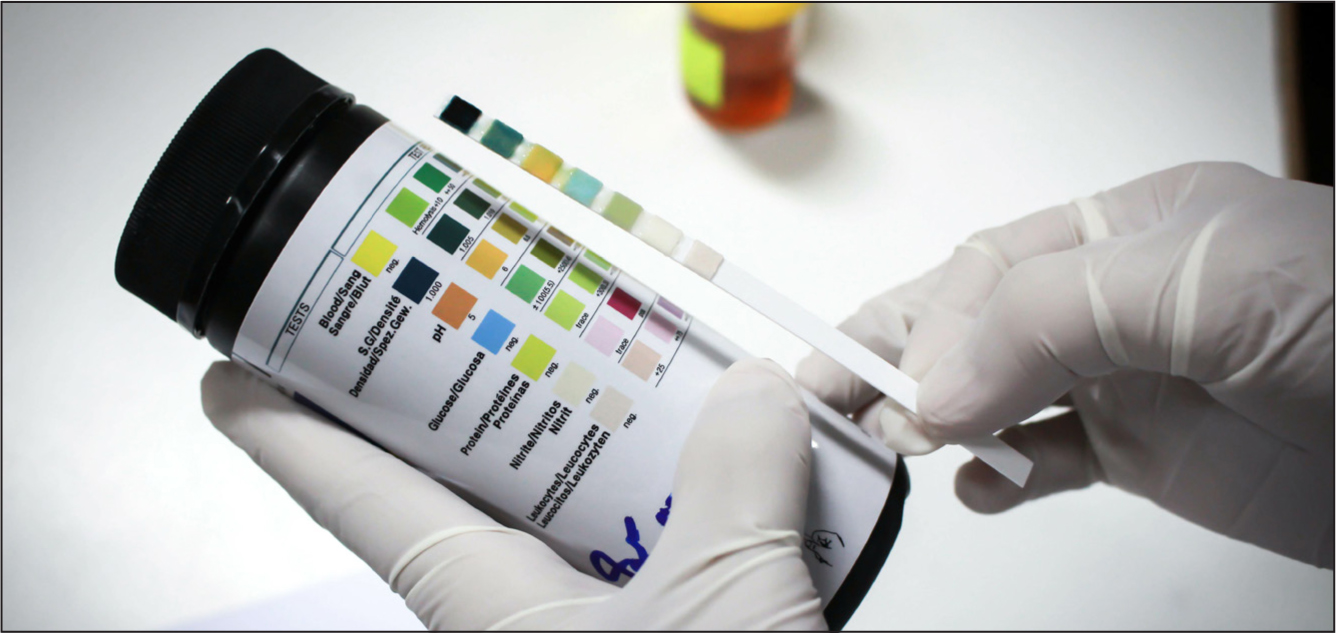
Specific gravity indicates the concentrating ability of the kidneys, with low values suggesting urine dilution through excessive diuresis. On the other hand, presence of protein in urine (proteinuria) is likely to be a consequence of damage to the glomeruli, often linked to chronic kidney disease (Table 2). The most common protein found in urine is albumin and the degree of albuminuria is usually assessed with the albumin–creatinine ratio (ACR) test (albumin mg / creatinine g) – this measurement is considered along with the GFR to categorise risk for kidney disease progression, morbidity and mortality (Seidu et al, 2020).
Table 2. Urine composition
| Components of urine | Normal adult values (usually measured in 24-hour collection specimen) |
|---|---|
| pH | 4.5–8.0 |
| Sodium | 27–287 mEq/litre/24 hours |
| Potassium | 25–123 mEq/litre/24 hours |
| Urea | 165–583 mmol/litre/24 hours |
| Creatinine | 500–2000 mg/24 hours |
| Uric acid | 1.4–4.5 mmol/24 hours |
| Phosphorus | 0.9–1.3 g/24 hours |
| Calcium | 100–250 mg/24 hours |
| Chloride | 110–250 mEq/litre/24 hours |
| Ammonia | 10 to 105 mEq in 24 hours |
| Water | 96% |
| Should not have: | |
|
|
The next step for urologic diagnosis is often timed urine collection – which may vary in duration from 2 to 24 hours – providing information on how the kidneys excrete and conserve various solutes.
Kidney biopsy
Biopsy might be required to investigate damage caused to the kidneys and aid diagnosis. A small piece of the kidney is taken away to be analysed under a microscope (Cooper and Gosnell, 2019).
Disorders and causes of renal system diseases
Kidney disease has many potential causes and disorders associated with it and prevalence varies by country, ethnicity, gender and age. The following are some of the most commonly seen in practice.
Cystitis
This is inflammation of the bladder, caused by bacteria (E. coli) entering the bladder through the urethra, in other words a urinary tract infection (UTI). Indicators of this in the physical examination and history taking will be:
- Frequency
- Urgency
- Pyuria (increased presence of white blood cells in urine)
- Dysuria
- Haematuria
- Nocturia
- Abdominal pain or discomfort
- Urinary incontinence.
The main investigation would be a midstream urine sample. In some cases a flexible cystoscopy (endoscopy of the urinary bladder via the urethra) may be required to detect abnormalities, and urine cytology to rule out renal cancer. Infection is treated with antibiotics and health education should be provided to reduce the likelihood of reinfection: personal hygiene education, especially in women, and regarding voiding urine before and after sexual intercourse. Patients would be advised to increase fluid intake unless contraindicated.
Renal calculi
Stones in the urinary tract are asymptomatic in the early stages, however, patients report colicky pain, haematuria, nausea and vomiting if stones are in the ureters. There is also a dull pain in the suprapubic region after voiding urine. Investigations would involve:
- Urine analysis to detect UTI and haematuria
- Abdominal X-ray (to identify urine obstruction)
- Intravenous pyelogram (shows position of stone)
- Blood tests – full blood count, urea and electrolytes
- Cystoscopy.
Interventions for urinary stones would include dietary modification if they are the result of excessive intake of calcium, protein, oxalates (found in chocolate, rhubarb and nuts) or vitamin D. The patient should be encouraged to drink fluids (2.5–3 litres a day), and to make lifestyle changes to include regular exercise to prevent urinary stasis. Patient education should cover recognising the signs of a UTI as well as advice regarding medication adherence, and providing information to reduce their anxiety. Pain management is another key intervention.
Acute pyelonephritis
This is an infection of the upper urinary tract involving both parenchyma and kidney pelvis. It usually starts as an infection of the lower urinary tract, progressing upwards to the kidneys. Patients who are pregnant, have indwelling catheters, diabetes, genitourinary tract abnormalities or immunosuppression are at increased risk of complications (National Institute for Health and Care Excellence (NICE), 2019a).
Symptoms usually develop within hours or over the course of a day (although it should be noted that in children symptoms can be absent).
- Flank pain
- Nausea or vomiting
- Dysuria and haematuria, especially in women
- Sudden onset of fever
- Suprapubic tenderness.
Investigations would be first of all a comprehensive history and physical assessment, including any previous medical history of UTI and kidney stones. Urinalysis is used to confirm diagnosis, with pyuria the most common finding. A urine culture is necessary to identify the micro-organism responsible and inform a decision on antibiotics. Blood tests would be a full blood count – raised white blood cells indicate infection, while renal markers (creatinine and urea) are used to assess any repercussions in kidney function. Imaging may be required: abdominal/pelvic CT scan with contrast for unwell septic patients, and potentally an ultrasound to reveal any renal abnormalities.
Whether the patient is managed as an outpatient or inpatient will depend on comorbidities and risk of deterioration. Inpatient management is usually required for elderly patients, those who are immunocompromised, have poorly controlled diabetes, or have previously had a renal transplant.
Pharmacological interventions would be:
- Antibiotics, with choice based on urine culture (oral or IV depending on setting) (NICE, 2019a)
- Analgesia (oral or IV)
- Antipyretics
- IV anti-emetics and fluids if there is dehydration due to nausea and vomiting
Non-pharmacological interventions would be the usual patient education regarding fluid intake and personal hygiene, recognising signs and symptoms of UTIs. Patients considered at lower risk and managed as outpatients will still need education on the signs of urosepsis and when to seek urgent assistance.
Acute kidney injury
The term acute kidney injury (AKI) describes an abrupt deterioration (within 48 hours) in filtration marked by increased serum creatinine (from baseline) with or without reduction in diuresis (KDIGO, 2012). AKI replaced outdated terms such as ‘acute renal failure’ or ‘acute renal insufficiency’, staging definitions for AKI are shown in Table 3.
Table 3. Stages of acute kidney injury (AKI)
| AKI stages | Serum creatinine changes | Urine output changes |
|---|---|---|
| 1 | >1.5–1.9 x the baseline | <05 ml/kg/hour for 6 hours |
| 2 | >2–2.9 x baseline | <0.5 ml/kg/hour for 12 hours |
| 3 | >3 x baseline | <0.3 ml/kg/hour for 24 hours or anuria (no urine production) for >12 hours |
Causes of AKI can be classified as:
- Prerenal: due to reduced kidney perfusion, often because of hypovolaemia, decreased cardiac output (Mercado et al, 2019)
- Intrarenal: damage to the kidney parenchyma (where waste excretion takes place) and nephrons usually due to nephrotoxic drugs or nephritis (Mercado et al, 2019)
- Postrenal: inadequate urine drainage along the ureters, bladder and urethra, commonly secondary to stones or prostate enlargement (Nagalingam, 2021).
Therefore the physical assessment should include assessment of volume status (pulse, blood pressure, including any postural changes, capillary refill time, jugular venous pressure, skin turgor). Peripheral oedema, chest auscultation and weight history will inform fluid status. Any abrupt changes in weight might suggest hyper-or hypovolaemia. Skin rashes can indicate systemic illness. Urine output should be assessed and, if an inpatient, review and monitoring of fluid charts should consider the different stages of AKI (Table 3).
Medication history can provide information on potential underlying causes – such as non-steroidal anti-inflammatory drugs (NSAIDs), angiotensin-converting enzyme (ACE) inhibitors, or some antibiotics. These are often referred to as ‘nephrotoxic’ drugs; however, Jones and Tomson (2018) argued that it is not helpful to describe ACE inhibitors and angiotensin II receptor blockers (ARBs) in this way, since the effect is reversible and in other contexts they have a protective effect, being used to preserve kidney function in patients with cardiovascular problems, hypertension and diabetes. The latest NICE (2019b) guideline avoids the term, instead referring to ‘drugs that can cause or exacerbate kidney injury’.
Investigations would cover (Rahman et al, 2012):
- Urinalysis (including ACR level)
- Full blood count (excluding underlying infection and/or anaemia)
- Renal profile bloods, including urea, creatinine, eGFR
- Imaging studies might inform whether obstruction is present (post-renal causes)
- Postvoid residual urine greater than 100 ml can suggest postrenal AKI.
Generally, AKI requires inpatient admission unless a clear reversible cause has been identified.
Pharmacological interventions for AKI include (Brochard et al, 2010; KDIGO, 2012):
- Stop any drugs that can cause or exacerbate kidney injury (in particular, metformin should be avoided) and adjustment of medications to renal dosages when applicable. Involve and liaise with pharmacists and prescribers
- Ensure volume status, which might require intravenous fluid (eg, normal saline)
- Maintain arterial blood pressure above 65 mmHg, which might require vasopressors if hypotension is present.
Non-pharmacological interventions should cover the following areas (KDIGO, 2012; NICE, 2021a):
- Avoid and monitor closely electrolyte imbalances (hyperkalemia, hyponatraemia, hypermagnesemia)
- Avoid and monitor hyperglycaemia closely
- Avoid radiocontrast procedures (such as angiography, CTCA) (because radiocontrast agents put stress on the kidneys)
- Patient education would be directed towards adequate hydration, regular follow-up and renal function monitoring, when to seek medical advice and how to avoid UTIs.
Psychosocial assessment should encompass psychological and social support – often this is the first time a patient may have come into contact with specialist health professionals who can provide support.
Chronic kidney diease
CKD refers to a persistent abnormality in the kidney structure or function for more than 3 months (KDIGO, 2013). It is recognised as a global public health problem, with an estimated global prevalence of 13.4% (KDIGO, 2013), representing a total cost to the NHS of around £1.4 billion in 2009/10 (Kerr, 2012; NHS England, 2022)
CKD is defined by GFR below 60 ml/minute/1.73 m2, and albuminuria of at least 30 mg/24 hours. It is classified in stages from 1 to 5 depending on GFR and albuminuria (Table 4)
Table 4. Risk categories for kidney disease progression, morbidity and mortality, based on albuminaria and glomerular filtration rate (GFR)
| Albuminuria categories | ||||||
|---|---|---|---|---|---|---|
| A1 | A2 | A3 | ||||
| Normal to mildly increased | Moderately increased | Severely increased | ||||
| <30 mg/g<3 mg/mmol† | 30–299 mg/g 3–29 mg/mmol† | ≥300 mg/g≥30 mg/mmol† | ||||
| GFR stages | G1 | Normal or high | ≥90* | |||
| G2 | Mildly decreased | 60–90* | ||||
| G3a | Mildly to moderately decreased | 45–59* | ||||
| G3b | Moderately to severely decreased | 30–44* | ||||
| G4 | Severely decreased | 15–29* | ||||
| G5 | Kidney failure | <15* | ||||
Key
▪ Low risk (if no other markers of kidney disease and CKD)
▪ Moderately increased risk
▪ High risk
▪ Very high risk
▪ Highest risk
*Glomerular filtration rate in ml/minute/1.73m2
†Urinary albumin–creatinine ratio
Source: Adapted from KDIGO, 2013During the physical assessment and history taking it is important to determine duration of kidney disease and if GFR has been below 60 for less than 3 months, then AKI on CKD is possible and tests should be repeated (KDIGO, 2013). Review and evaluate volume status; hypovolaemia suggests overdiuresis, whereas hypervolaemia is often linked to liver, heart failure or nephrotic syndrome (Chen et al, 2019)
CKD is usually identified through routine renal profile bloods, although less commonly patients present with the following symptoms (Chen et al, 2019):
- Lethargy, fatigue
- Headache
- Breathlessness
- Peripheral oedema
- Proteinuria, hematuria, nocturia
- ‘Foamy urine’ (sign of albuminuria)
- Oliguria
- Anuria
- Symptoms of anaemia
- Poor appetite, nausea, or vomiting,
- Weight loss
- Pruritus (itchy skin).
Investigations would include renal function blood tests (serum creatinine and GFR, and full blood count to identify the extent of any anaemia. Urine samples would be needed for urine ACR and analysis for specific gravity, as well as a urine culture (to test for UTI). Advanced cases of CKD may require renal biopsy, and renal ultrasound is sometimes needed to determine whether there is an obstruction, or otherwise help in identifying the aetiology of CKD (NICE, 2021b).
Pharmacological interventions for CKD would be, first, to avoid drugs that cause or exacerbate kidney injury (eg ACE inhibitors, NSAIDs and certain herbal remedies). Adjustment of drug dosing is frequently required on medications such as antibiotics, oral anticoagulants, and hypoglycaemic agents, among others (Chen et al, 2019).
Non-pharmacological interventions should consider the following (Think Kidneys, 2017):
- Referral to specialist teams for regular follow up (nephrology)
- Promote healthy lifestyle (low salt diet, low potassium diet, regular exercise, avoidance of medications that can damage the kidneys, low alcohol)
- Encouragement and empowerments towards self-care (such as signs and symptoms of worsening renal function, monitor fluid intake and output)
- Address co-morbidities such as hypertension, anaemia, low calcium or phosphate and diabetes
- Offer psychosocial interventions.
Case study
Presenting complaint
Nile presented to his GP surgery for an annual routine renal function review and following assessment, his eGFR result was 65 ml/minute/1.73 m2 and his ACR was 5 mg/mmol. His eGFR result has not changed from his result in the past 2 years.
Social history
Nile is a widowed 70-year-old gentleman, who lives alone in sheltered accommodation, smoking 20 cigarettes a day. He drinks 2 cans of beer a day to help with daily boredom when his friends are not available to meet him. His mobility is mildly reduced, mobilising with support of a stick and ‘furniture walking’ around the house.
Prior medical history
- Chronic hypertension, managed with losartan 25 mg once daily
- Chronic kidney disease, diagnosed a year ago
- Type 2 diabetes, controlled by gliclazide 30 mg once daily
- Recent urinary tract infections (3 in the last 6 months).
Subjective and objective assessment
- Recent onset of nocturia in the past few days. Reports urine had a ‘foul’ smell last week
- Peripheral and bilateral ankle oedema, worse during the day
- Weight gain of 2 kg in the past 2 days, above his known ‘dry’ and usual weight. (In renal care, dry weight is weight without the excess fluid that builds up between dialysis treatments. This weight is similar to what a person with normal kidney function would weigh after urinating).
Care plan
The nurse involved in Nile's case recognises the complexity of his needs, requiring involvement including psychosocial practitioners, dietitians, pharmacists, social workers, nephrologists, and specialist nurses to provide holistic care. Ultimately this will lead to better health-related outcomes, such as lower hospitalisation rates, slower CKD progression and reduced risk of mortality (Collister et al, 2019).
Causes of CKD, in this case, are multi-factorial, including chronic hypertension, type 2 diabetes mellitus and lifestyle behaviours (such as sedentarism from low mobility and alcohol consumption) (Peng et al, 2019). Fears related to future consequences and disease trajectory are commonly described by renal patients and patients' own illness perceptions should be addressed regularly during routine follow-up (Clarke et al, 2016).
The clinical care plan in Table 5 focuses on interventions that preserve renal function, and prevent adverse effects of comorbidities, however, they come at the cost of requiring several behavioural changes in Nile's daily routine. Effective self-management requires active patient participation; however, the degree of willingness to engage with these strategies can vary (Donald et al, 2018). The emotional burden associated with therapeutic goals and lifestyle changes is known to lead to non-concordance with self-management strategies (Welch et al, 2015).
Table 5. Clinical care plan for Niles
| Self-management empowerment |
|---|
Address mental wellbeing and psychosocial implications
|
| Manage comorbidities |
Blood pressure management
|
| Polypharmacy/medication regimens |
|
Conclusion
Renal biomarkers are essential to guide management plans, prognosis, and disease trajectory. However, good history taking and physical assessment remain essential to differentiate between the wide range of renal disorders. Serum creatinine and GFR are often used and requested in blood tests across clinical practice, therefore nurses must have an understanding of what these results mean and how to plan care accordingly.
Acute kidney injury and chronic kidney disease are public health problems. Care plans require an integrated and multidisciplinary team approach. Interventions should focus equally on both physical health and mental wellbeing. The emotional burden associated with demanding lifestyle changes has been well described in qualitative studies focusing on renal patients. Empowerment towards self-management, and addressing comorbidities, are essential not only to reduce mortality and morbidity but also to achieve better patient-directed outcomes such as quality of life and satisfaction with care. Adequate follow-up requires a patient-centred approach, with careful consideration in the development of tailored and achievable care plans. Active participation is required from both patients and professionals, as patients' needs, and perceptions of illness change throughout the renal disease trajectory.
KEY POINTS
- The renal system is an important system in the body and plays a key role in homeostasis
- When not managed, renal disease can progress to end-stage kidney disease
- Nursing care for this comorbid group of patients can be challenging as patients can deteriorate rapidly
- Nurses need an understanding of the different classifications and interventions to be able to provide patient-centered care
CPD reflective questions
- How is renal disease assessed in your clinical area?
- What support is in place to promote healthy behaviours for patients with renal disease?
- What support is provided for patients with end-stage kidney disease


